Alexandre Antônio Marques Rosa1; Thais Sousa Mendes2; Paulo Sérgio Miranda de Oliveira Filho3
DOI: 10.17545/eoftalmo/2018.0013
ABSTRACT
The authors report a case of non-surgical repositioning of a fragment of a dexamethasone implant that migrated to the anterior chamber. A 66-year-old male with aphakia and a history of cataract surgery evolving with dislocation of the lens to the vitreous cavity. Optical coherence tomography (OCT) revealed cystoid macular edema, with the therapeutic indication of an intravitreal dexamethasone implant. On biomicroscopy, he presented a fragment of the implant in the inferior anterior chamber. The standard treatment is surgical repositioning. However, an extrinsic ocular mobilization technique was used, which successfully non-surgical replacement of the implant to the vitreous cavity.
Keywords: Dexamethasone; Anterior Chamber; Ophthalmologic Surgical Procedures; Eye manifestations.
RESUMO
Os autores relatam um caso sobre o reposicionamento não cirúrgico de um fragmento de implante de dexametasona, o qual migrou para a câmara anterior. Paciente de 66 anos, masculino, afácico, com histórico de cirurgia de catarata, evoluindo com luxação de lente para cavidade vítrea. Ao exame de OCT, foi constatado edema macular cistoide, com indicação terapêutica de implante intraocular dedexametasona. À biomicroscopia, apresentava um fragmento do corticoide posicionado na câmara anterior, inferiormente. O tratamento padrão é o reposicionamento cirúrgico, entretanto, utilizamos uma técnica de movimentação ocular extrínseca, a qual recolocou com sucesso o fragmento para a cavidade vítrea.
Palavras-chave: Dexametasona; Câmara Anterior; Procedimentos Cirúrgicos Oftalmológicos; Manifestações Oculares.
RESUMEN
Los autores relatan un caso sobre el reposicionamiento no quirúrgico de un fragmento de implante de dexametasona, lo cual migró a la cámara anterior. Paciente de 66 años, masculino, afásico, con histórico de cirugía de catarata, evolucionando con luxación de lente para cavidad vítrea. En el examen de OCT, se constató edema macular cistoide, con indicación terapéutica de implante intraocular de dexametasona. Según la biomicroscopía, presentaba un fragmento del corticoide posicionado en la cámara anterior, inferiormente. El tratamiento estándar es el reposicionamiento quirúrgico, sin embargo, utilizamos una técnica de motilidad ocular extrínseca, la cual recolocó con éxito el fragmento en la cavidad vítrea.
Palabras-clave: Dexametasona; Cámara Anterior; Procedimientos Quirúrgicos Oftalmológicos; Manifestaciones Oculares.
INTRODUCTION
Dexamethasone ocular implants (Ozurdex®, Allergan, Inc., Irvine, CA) are rod-shaped, made of a biodegradable polymer, and 6-mm long and 0.46-mm wide and contain 0.7 mg of the drug, which is gradually released into the posterior chamber of the patient’s ocular globe over months.1,2 It is indicated in cases of macular edema associated with retinal vein occlusions, non-infectious posterior uveites, and diabetic macular edema.1,2,3
Cataract formation, increased intraocular pressure (IOP), and secondary glaucoma are possible complications from the use of this medication.1 IOP is defined by the balance between the production and drainage of aqueous humor, and it varies according to anatomical and physiological factors. The value considered normal for tonometry varies from 11 to 21 mmHg. These numbers can fluctuate during the day, influenced by various factors, such as arterial pressure and even breathing.4
The main pathology associated with high IOP as a risk factor is glaucoma, whose symptoms are associated with the progressive loss of the visual field due to damage to retinal ganglion cells. However, patients with IOP parameters fluctuating within the normal range can also develop glaucoma because it is a multifactorial disease. Processes such as alterations in retinal microcirculation, changes in immunity and oxidative stress are possible factors that trigger glaucoma.4,5
Glaucoma affects approximately 3% of the population aged more than 40 years, a number estimated at 70 million patients worldwide. In Brazil, it is estimated that there are approximately 985,000 patients with glaucoma aged more than 40 years, 70% of whom have not yet been diagnosed.4,5,6
Some reports show the possibility of segmentation/breakage of the implant inside the ocular cavity at the time of application, which mainly occurs due to manufacturing defects.4 There is also a chance of migration of the implant to the anterior chamber in patients with aphakia, which can lead to the development of corneal edema with associated endothelial injury and, consequently, increased IOP.7,8,9
The management of implant dislocation is essentially surgical to avoid complications from the presence of the medication in the anterior segment. However, in this report we describe the relocation of an implant fragment that had migrated to the anterior chamber, using a simple, non-surgical technique of ocular mobilization.
CASE REPORT
A 66-year-old male (initials V.P.S.) from Belém, State of Pará, Brazil, referred for ophthalmological evaluation with a complaint of poor visual acuity in the right eye for the past 5 months.
Personal history had no clinical relevance. Ocular history was as follows: the patient had a history of cataract surgery in the right eye with complications (August 2014), evolving with dislocation of the intraocular lens to the vitreous cavity, which later required a posterior vitrectomy. Subsequently, an anterior-chamber intraocular lens was surgically implanted (September 2014).
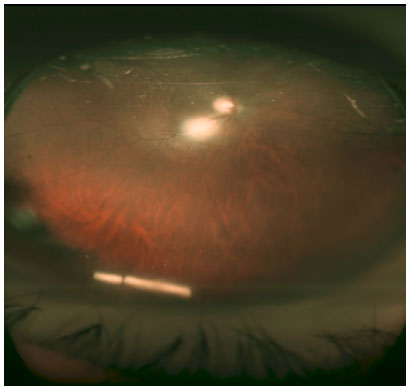
Biomicroscopy examination revealed an intact cornea, a regular pupil, and a well-positioned anterior chamber intraocular lens (Figure 1). On retinal examination, we observed a 0.5 papillary excavation with foveal thickening of petaloid aspect.

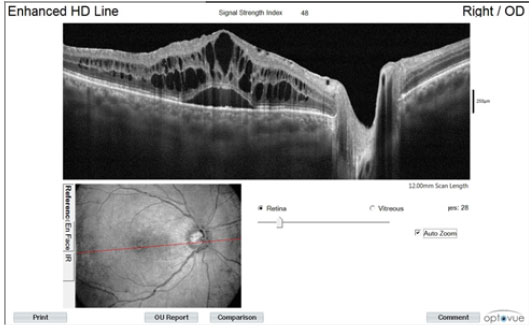
In the initial (OCT) assessment, we observed cystoid macular edema (Figure 2).
A dexamethasone implant in the right eye was indicated and performed without complications. In the postoperative room, the patient complained of seeing a foreign body in front of the eye. In the biomicroscopy examination, we observed an intact cornea, with a polymer fragment in the inferior portion of the anterior chamber (Figure 1). The patient was positioned in dorsal horizontal decubitus and a maneuver was performed to reposition the polymer (Video 1), which was successful, as we can see in the retinography (Figure 3) with the polymer fragment once again inside the vitreous cavity located inferiorly.
The goal of the ocular mobilization maneuver was to spare the patient from an invasive approach.
In an outpatient consultation room, the patient was positioned in dorsal decubitus under good lighting. Initially, the right eye was mobilized superiorly, with a posterior movement in the nasal direction followed by superior-medial positioning. The eye was repositioned superiorly, followed by a lateral movement, and subsequently returned to the superior position. The patient was instructed to move the eye inferiorly and position it superiorly on two subsequent occasions. At this point, the fragment had been successfully repositioned to the posterior chamber. After 7 days, the implant fragment remained in the vitreous cavity.
DISCUSSION
Most common indications for using the Ozurdex® implant are macular edema associated with retinal vein occlusions or non-infectious posterior uveites and diabetic macular edema.1,2,3 Its use is also described in patients with cystoid macular edema following cataract surgery, also called the Irvine–Gass syndrome.10
Corticosteroids play a key role in the treatment of retinal disease, particularly in cystoid macular edema, which results from the breakdown of the bloodretinal barrier, accompanied by an increase in inflammatory mediators. In patients with diabetes, the focus is on the suppression of leukocyte adhesion, which leads to a decrease in protein levels, less overload on the hematoretinal barrier, and therefore less endothelial growth factor (VEGF) activity.11
As we saw in the patient described in the present report, the implant was indicated because of cystoid macular edema that was confirmed in the consultation, with a possible postsurgical etiology of complications from cataract surgery and anterior chamber lens implant, which can be responsible for causing subclinical uveitis and an increase of inflammatory products, which in turn are responsible for injuring the corneal endothelium.12
The implant is performed by a microsurgery technique, using an applicator pre-loaded with the biodegradable rod-shaped medication. The possibility of implant breakage is associated with manufacturing problems, although there is the possibility of breakage with the normal process of wear. According to the manufacturer, in cases of breakage, there are no changes in the pharmacokinetics, time of action, or expected effects of the medication.8
Another complication is the dislocation of the dexamethasone implant to the anterior chamber of the eye, which is uncommon, even in the presence of an intraocular lens, with a higher incidence in patients with aphakia and pseudoaphakia. Patients with a history of vitrectomy are also at higher risk.9
To prevent this dislocation during follow-up, the patient should avoid physical exertion, prone or face-down positions, and flights for long duration, because the difference in pressure between the body and the airplane may dislocate the implant to the anterior chamber.7
This dislocation is associated with the development of corneal edema, which is the main complication. The mechanism observed is due to the toxicity of the degradation of the drug into lactic acid or glycolic acid. Trauma from the presence of a foreign body cannot be ruled out.9 Specular microscopy shows the loss of corneal endothelial cells in cases of implant migration. However, it is known that the use of low doses of dexamethasone is well tolerated in the treatment of inflammation following cataract surgery.13
It is also known that approximately 12% of the patients in the GENEVA study developed ocular hypertension following a single implant treatment. However, the Shasta study shows that only 1.7% of the patients with increased IOP due to the use of the medication will undergo surgery for glaucoma, which indicates that the effect of the corticosteroid in increasing the IOP is transitory.14
The implant is repositioned surgically in some cases, but it is possible to use a 30G needle and a slit lamp under local anesthesia to reposition the implant to the posterior chamber. These techniques reduce the need for surgical intervention, reducing the risk inherent in the procedure.7,15,16
In the present case, there were two unusual occurrences: the breakage of the implant into two pieces and the dislocation of one of the fragments into the anterior chamber. The patient had the risk factors for this mobilization, i.e., anterior chamber lens for aphakia and prior vitrectomy, although the presence of these factors does not necessarily mean that dislocation will occur. We observed corneal edema with Descemet’s folds from the patient’s delay in returning to the clinic. In this case, the non-surgical implant repositioning option was chosen, as it would reduce patient risk, associated with the fact that dislocation of only one of the fragments had occurred, which we believe to have facilitated the success of the non-surgical repositioning.
CONCLUSION
Dexamethasone implants present low patient risks when compared to the benefits of treatment. However, the possibility of dislocation of the medication to the anterior chamber, particularly in cases of patients with aphakia with implant fragmentation and prior vitrectomy, cannot be ignored. The indicated treatment is surgical removal, but in cases like the one presented, it is possible to relocate the implant fragment using an ocular movement technique, thereby preventing corneal edema and increased IOP.
REFERENCES
1. Haller JA, Bandello F, Belfort R Jr., Blumenkranz MS, Gillies M, Heier J, et al.; OZURDEX GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-6.e3.
2. Lowder C, Belfort R Jr., Lightman S, Foster CS, Robinson MR, Schiffman RM, et al.; Ozurdex HURON Study Group. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545-53.
3. Pacella E, La Torre G, Turchetti P, Merisola C, Lenzi T, Mazzeo F, et al. Evaluation of efficacy dexamethasone intravitreal implant compared to treatment with anti-VEGF in the treatment of diabetic macular edema. Senses Sci. 2014;1(4):164-8.
4. Salmon J, Bowling B. Kanski’s Clinical Opthalmology: A Systematic Approach. 8th ed. Shanghai: Elselvier; 2015.
5. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-11. DOI: 10.1001/jama.2014.3192.
6. Gonçalves MR, Guedes MMR, Chaves MAPD, Pereira CCL, Otton R. Análise dos fatores de risco e epidemiologia em campanha de prevenção da cegueira pelo glaucoma em João Pessoa, Paraíba. Rev Bras Oftalmol. 2013;72(6):396-9.
7. Pacella F, Agostinelli E, Carlesimo SC, Nebbioso M, Secondi R, Forastiere M, et al. Management of anterior chamber dislocation of a dexamethasone intravitreal implant: a case report. J Med Case Rep. 2016;10(1):282.
8. Agrawal R, Fernandez-Sanz G, Bala S, Addison PK. Desegmentation of Ozurdex implant in vitreous cavity: report of two cases. Br J Ophthalmol. 2014;98(7):961-3.
9. Khurana RN, Appa SN, McCannel CA, Elman MJ, Wittenberg SE, Parks DJ, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121(1):67-71.
10. Carricondo PC, Abalem MF, Machado CG, Kara-Junior N. Profilaxia e tratamento do edema macular cistoide após cirurgia de catarata. Rev Bras Oftalmol. [Internet]. 2015 Apr [cited 2018 Feb 01]; 74(2):113-8. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S003472802015000200113&lng=en. DOI: http://dx.doi.org/10.5935/0034-7280.20150026
11. Dias JRO, Nunes RP, Goldhardt R. New Drugs and New Posterior Delivery Methods in CME. Curr Ophthalmol Rep. 2017;5:160-8. DOI: https://doi.org/10.1007/s40135-017-0134-3
12. Ahmad M, Naeem M, Iqbal S, Khan S. Visual Outcome and Complications of Anterior Chamber Intraocular Lens Versus Scleral Fixated Intraocular Lens. Pak J Ophtalmol. 2012;28(4):206-10.
13. Rahimy E, Khurana RN. Anterior segment migration of dexamethasone implant: risk factors, complications, and management. Curr Opin Ophthalmol. 2017;28(3):246-51.
14. Kapoor KG, Wagner MG, Wagner AL. The Sustained-Release Dexamethasone Implant: Expanding Indications in Vitreoretinal Disease. Semin Ophthalmol. 2015;30(5-6):475-81.
15. Kishore SA, Schaal S. Management of anterior chamber dislocation of dexamethasone implant. Ocul Immunol Inflamm. 2013;21(1):90-1. DOI: 10.3109/09273948.2012.736589
16. Bansal R, Bansal P, Kulkarni P, Gupta V, Sharma A, Gupta A. Wandering Ozurdex(®) implant. J Ophthalmic Inflamm Infect. 2012;2(1):1-5. DOI: 10.1007/s12348-011-0042-x


Funding: No specific financial support was available for this study.
CEP Approval: Not applicable.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
March 20, 2018.
Accepted on:
April 6, 2018.