André Mózena1; Guilherme Henrique Tanajura2; Maria de Fátima de Carvalho Ferreira3; Jair Giampani Júnior2; Marcial Francis Galera3,4
DOI: 10.17545/eOftalmo/2023.0009
ABSTRACT
PURPOSES: To identify ophthalmological changes in children with congenital Zika virus syndrome residing in the city of Cuiabá; to correlate these findings with congenital Zika virus infection; and to present the clinical profile of the mothers of those children.
METHODS: This study was an observational, descriptive case series.
RESULTS: A total of 29 children were examined between March 2019 and March 2020. Of those, three were excluded after diagnostic confirmation of cytomegalovirus during the medical record review. Of the remaining 26 children, 15 and 11 were females and males, respectively. The average age of the children was 2.21 ± 0.79 years. Most mothers (57.69%) reported symptoms during pregnancy. Microcephaly was present in 73.08% of the children (19/26); among them, some degree of structural alteration was observed in 55.26% of the examined eyes. The average spherical equivalent of the sample was +0.49 ± 2.13 diopters. The average intraocular pressure was 14 ± 2.59 mmHg. The mean corneal diameter was 11.36 ± 0.72 mm. The average central corneal thickness was 568.33 ± 46.21 µm. The mean anteroposterior diameter was 21.08 ± 1.21 mm.
CONCLUSION: The frequency of ophthalmic effects induced by Zika virus was similar to that reported in other studies. Fundus changes were found in 46.15% of the examined eyes. Other ocular abnormalities (e.g., refractive errors, nystagmus, and strabismus) were also highly prevalent. The ocular biometric data (i.e., corneal diameter, anteroposterior diameter, central corneal thickness) in these children were normal in relation to the healthy population of the same age group.
Keywords: Ophthalmology; Eye manifestations; Microcephaly; Congenital Zika syndrome; Congenital Zika virus infection.
RESUMO
OBJETIVO: Identificar alterações oftalmológicas em uma amostra de crianças com síndrome congênita por zika vírus do município de Cuiabá, correlacionar esses achados com a infecção congênita por zika vírus e apresentar o quadro clínico apresentado pelas mães das crianças avaliadas.
MÉTODOS: Este estudo é um relato de série de casos, observacional, descritivo.
RESULTADOS: Foram examinadas 29 crianças entre março de 2019 e março de 2020, das quais 3 foram excluídas após confirmação diagnóstica de citomegalovirose durante a revisão de prontuário. A idade média das crianças foi de 2,21 ±0,79 anos. Quinze das 26 crianças eram do sexo feminino. A maioria das mães (57,69%) referiu sintomas durante a gestação. Microcefalia esteve presente em 73,08% das crianças (19/26) e, desses pacientes, 55,26% de seus olhos apresentou algum grau de alteração estrutural. A média de equivalente esférico da amostra foi de +0,49 ± 2,13 dioptrias. A pressão intraocular média foi de 14 ± 2,59 mmHg. A média do diâmetro corneal foi de 11,36 ± 0,72 mm. A espessura de córnea central mostrou valor médio de 568,33 ± 46,21 µm. O diâmetro ântero-posterior médio foi de 21,08 ± 1,21 mm.
CONCLUSÃO: A frequência de achados oftalmológicos por síndrome congênita por Zilka Virus foi semelhante à de outros estudos. Alterações fundoscópicas foram encontradas em 46,15% dos olhos examinados. Outras anormalidades oculares também foram muito prevalentes, como erros refrativos, nistagmo e estrabismo. Os dados biométricos oculares (diâmetro corneal, diâmetro ântero-posterior, espessura da córnea central) foram normais em relação à população saudável da mesma faixa etária.
Palavras-chave: Oftalmologia; infecção por Zika vírus; manifestações oculares; Microcefalia; síndrome congênita de Zika; Doença por Zika vírus.
INTRODUCTION
Zika virus (ZKV) is a RNA virus of the Flaviviridae family (Flavivirus genus). It is related to the dengue, chikungunya, yellow fever, and West Nile fever viruses. This virus was first identified in the 1940s in Zika Forest, Uganda1,2. In 1952, the ZKV was isolated in humans in Uganda and Tanzania1. The main vector of urban transmission is the Aedes aegypti mosquito. Transmission of the virus through numerous routes (i.e., perinatal transplacental, breastfeeding, sexual intercourse, blood transfusion, and organ transplantation) has been confirmed3-5.
Despite its wide geographical distribution in Africa and Asia, until 2007, there had been only sporadic reports of cases of ZKV infection, without major repercussions in the medical and social environment6.
The first major outbreaks of ZKV infection occurred in 2007 and 2013 in the Yap7 islands and French Polynesia8, respectively. Since then, there have been reports of ZKV infection in several South Pacific islands9, in Japan, Australia, Germany, France, and Norway. This evidence demonstrates the ability of ZKV to spread to non-endemic areas, in which the vector mosquito may be present10.
The first cases of ZKV infection in Brazil were identified in 2015, in the State of Bahia. However, the highest number of cases was recorded in the State of Pernambuco11. It was estimated that 1 million Brazilians were infected that year12. Concomitantly, a 20-fold increase in cases of congenital microcephaly was observed, totaling 1,248 cases in 2015, with a prevalence of 99.77 per 100,000 live births. ZKV was detected in the amniotic fluid of pregnant women with fetuses exhibiting microcephaly and in tissue obtained from a newborn with microcephaly who expired after birth13. Consequently, a causal relationship between maternal ZKV infection and microcephaly was theorized14.
The pathophysiology of lesions in infants remains incompletely understood; however, it may be associated with the direct action of the virus and/or a viral toxin, leading to an inflammatory reaction. This could result in known abnormalities, including eye damage14. Studies suggested that ZKV has a neurotropism for human neural progenitor cells, which may explain the more frequent detection of neurological changes in fetuses versus adults15-17.
Singh et al.18 hypothesized that ZKV, as a blood-borne virus, would initially reach the cells of the external and internal hematoretinal barriers. The cells would express receptors for the entry of the virus, as well as allow intracellular replication, thereby serving as reservoirs and facilitating viral spread to other organs.
The symptoms occurring in adults resemble those of a dengue-like condition, including rash, low-grade fever, myalgia, non-purulent conjunctivitis, arthralgia, pruritus, and malaise. They are often self-limited and persist for approximately 4-7 days19. However, most adults are asymptomatic9.
The abnormalities attributed to the congenital ZKV syndrome (CZS) are serious, including congenital microcephaly, craniofacial disproportion, spasticity, epileptic seizures, irritability, brain stem dysfunction (e.g., swallowing problems), arthrogriposis, hearing and eye abnormalities, and early pyramidal and extrapyramidal symptoms. Brain abnormalities are very commonly detected through imaging examination; such abnormalities include cortical and subcortical calcifications, cortical malformations, hypoplasia of the brainstem and cerebellum, ventriculomegaly, neuronal migration disorders, and simplified pattern of brain gyrations20-23.
Ocular findings occur in up to 50% of children with microcephaly due to CZS1. Ophthalmological changes already described in patients with microcephaly due to CZS are macular and peripheral chorioretinal atrophy, pigmentary changes in central and peripheral retina, vascular attenuation, optic atrophy, hypoplasic optical disc, increased cup-to-disc ratio, iris coloboma, and lens subluxation.
Subsequent studies24 found that the appearance and severity of ocular findings are directly related to the fetal development stage during which the infection occurs. Thus, infections that occur during the first and second trimester of pregnancy tend to cause more frequent and serious eye changes20. Other factors (viral load, the immune response of the mother and fetus, etc.) may play important roles in changes observed in newborns17-22. Therefore, the severity of maternal infection may directly influence the severity of infection in the fetus or newborn23.
Knowledge of ocular biometric data, the refractive state, and the mechanisms involved in changes with advancing age is essential for understanding eye growth and the development of eye diseases24-25. However, thus far, there are few population-based studies with normative information on biometric data of the eye and concerning eye refraction in children26,27.
In this study, we present the ophthalmological characteristics of children with CZS, as well as clinical data from their mothers.
METHODS
This research study was an observational, descriptive case series. The investigation was conducted in adherence with the tenets of the Declaration of Helsinki.
A sample of children with confirmed or suspected CZS from March 2019 to March 2020 was used. These patients were evaluated at the Ophthalmology outpatient clinic of Hospital Universitário Júlio Müller (HUJM) at the Universidade Federal de Mato Grosso (UFMT) (Cuiabá, Brazil).
The diagnostic criterion for confirmed or suspected cases of maternal ZKV infection was based on clinical and/or laboratory conditions (i.e., serology, reverse transcription-polymerase chain reaction). The diagnostic criteria for CZS were the presence of a compatible clinical and radiological profile after ruling out other infections, positive serological and molecular tests for ZKV, as well as clinical characteristics reported by mothers during pregnancy. Children with a head circumference measurement of less than two standard deviations for gestational age and sex, measured in the first 48 hours of life, were considered microcephalic21.
After directed anamnesis, anterior segment biomicroscopy was performed using a Topcon® slit lamp (Topcon Corporation, Japan). Strabismus research testing and intrinsic eye motility examination using a focal light source were also performed.
In sequence, cycloplegia was initiated shortly before referral to the operating room, where the patients were sedated to perform refraction. Subsequently, intraocular pressure (IOP) was measured using a Perkins® tonometer (Clement Clarke International Limited, UK), horizontal and vertical corneal diameters were determined using an ophthalmic compass, and afferent pupillary defect (APD) and central corneal thickness (CCT) were evaluated with an AL-4000 Bio & Pachymeter® (Tomey Corporation, Japan). In addition, indirect binocular fundoscopy was carried out.
Refraction values under cycloplegia28,29 were transformed into their corresponding spherical equivalents (SE). Refractive error was defined as SE ≤−0.75 diopters (D) or ≥+1.50 D. Anisometropia was defined as a difference in SE between eyes of ≥1.00 D.
RESULTS
Of the 29 examined children, three were excluded after diagnostic confirmation of cytomegalovirus during the medical record review. The remaining 26 children with confirmed or presumed diagnosis of CZS were evaluated. The mean age was 2.21 ± 0.79 years (range: 8 months to 4 years). Of those, 15 and 11 were females and males, respectively.
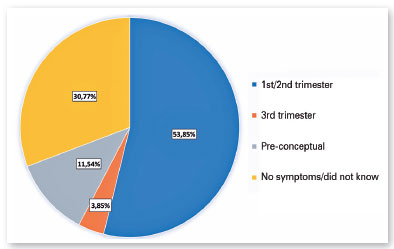
Based on the symptoms perceived by the mothers, Zika infection during pregnancy probably occurred during the first or second trimester in 53.85% of cases (14/26) and during the third trimester in 3.85% of cases (1/26); in the remaining eight cases (30.77%), it was not possible to estimate the time of infection. Unexpectedly, 11.54% (3/26) of the patients’ mothers reported symptoms in the pre-conceptual period (Figure 1).
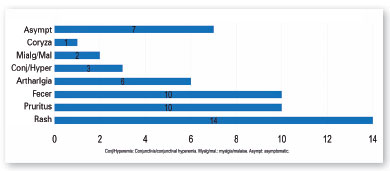
The symptoms most commonly reported by mothers were rash, pruritus, fever, and arthralgia (Figure 2). Three of the mothers reported symptoms perceived in the pre-pregnancy period.
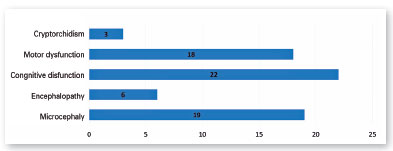
Nineteen children (73.08%) had microcephaly; 23.08% (6/26) had some other type of encephalopathy (i.e., intracranial calcifications, reduced brain volume, ventriculomegaly, agenesis of the corpus callosum, schizencephaly with polymicrogyria); 84.62% (22/26) had some degree of cognitive dysfunction; and 69.23% (18/26) had some degree of motor dysfunction (Figure 3).
Regarding intrinsic eye movement, three children showed decreased consensual and direct photomotor reflexes in both eyes; in two of them, these effects were associated with the presence of medium mydriasis. One child exhibited anisocoria and decreased direct photomotor reflex unilaterally. Left eye (oculus sinister [OS]) corneal punctate keratopathy and blepharitis (one case each) were also identified.
The refraction performed under cycloplegia showed a spherical equivalent average of +0.49 ± 2.13 D (-10.00 to +6.50 D) for the 52 eyes of the 26 children examined. Of the 52 eyes, 28 (53.85%) presented SE within the range of −0.50 D and +1.25 D. Eleven and 13 eyes were characterized as myopic and hyperopic, respectively. In addition, two patients had anisometropia.
The mean IOP was 14 ± 2.59 mmHg (range: 9-19 mmHg) in both eyes (oculi uterque [OU]), measured in the morning (8:00-10:30 am).
Regarding fundus findings, half of the children had some alteration (i.e., 10 in OU and three in only one eye). Therefore, of the 52 eyes, 23 (44.23%) showed fundus changes. When considering only patients with microcephaly (19/26), this percentage increased to 55.26% (21/38) of the examined eyes.
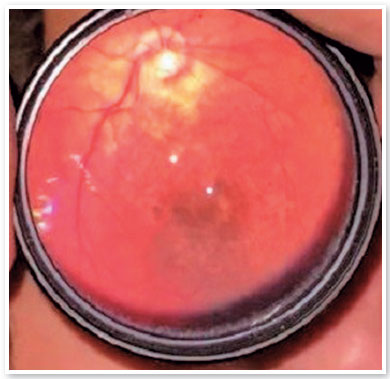
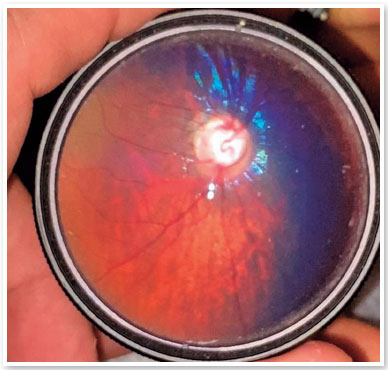
Cicatricial lesions of macular chorioretinitis appeared in 7.69% of the eyes (4/52). One eye (1.92%) had a chorioretinitis scar in the retinal periphery. Changes in the retinal pigment epithelium in the macula or retinal periphery were found in 15.38% of the eyes (8/52) (Figure 4). Changes in the optic disc, such as increased excavation and temporal quadrant pallor (unrelated to macular chorioretinitis scarring), occurred in 21.15% (11/52) of the eyes (Figure 5). In particular, only two children had increased papillary cupping (child BVCS: 0.8-0.9 OU; and child MRFC: 0.7 OU). Of those, child BVCS had an IOP of 19 mmHg OU; the CCT was 710 µm in oculus dexter (OD) and 706 µm in OS; and both the APD and corneal diameters were normal. Child MRFC had a CCT of 559 µm in OD and 567 µm in OS. However, the IOP was 11 mmHg and 10 mmHg in OD and OS, respectively. The other children had papillary excavations ≤0.6. The fundus examination did not reveal any alteration in half of the eyes (26/52).
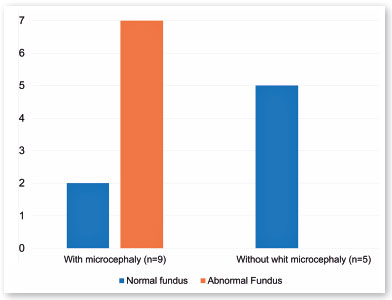
Fourteen children had intrauterine ZKV infection during the first or second trimester. Half of them (7/14) presented microcephaly associated with fundus abnormalities, equivalent to 13 of the 28 eyes. However, of those 14 children, five did not have microcephaly and had normal results in the fundus examination (Figure 6).
The mean corneal diameter was 11.36 ± 0.72 mm (range: 10.00-13.00 mm).
The CCT showed an average value of 568.33 ± 46.21 µm (range: 482.00-710.00 µm) for OU. The mean APD was 21.08 ± 1.21 mm (range: 18.36-24.19 mm). These values are presented in Table 1.
Other changes were identified through the eye examination. Attention was drawn to the presence of nystagmus and changes in eye alignment. In the present study, five of the 26 children (19.23%) had nystagmus; of those, four had intrauterine ZKV infection in the first or second trimester of pregnancy. Strabismus was present in 53.85% (14/26) of the children; in seven of those, infection occurred during the first two pregnancy trimesters.
DISCUSSION
The symptoms developing in adults are not specific to ZKV infection. The clinical profile of ZKV infection is similar to that of dengue (dengue-like) and may occasionally resemble an upper airway infection. Consequently, most infections are not noticed by the affected patients. Furthermore, the majority of patients are asymptomatic or oligosymptomatic1. The incidence of symptoms in patients’ mothers in this study was higher than those described in the literature. The remarkable variation in these rates was attributed to the diagnostic difficulty caused by the similarity of ZKV infection symptoms with those of other arboviruses and flu-like conditions. This higher incidence rate may also reflect a tendency for CZS to occur more frequently in symptomatic mothers (i.e., the majority of cases in this study; 57.69%). Another possible reason for this difference may be the intensified screening and monitoring of cases of ZKV infection in Brazil since 2015.
All 26 children examined in the study had some clinical abnormality. Nineteen of them (73.08%) had microcephaly. In a similar context, Ventura et al. also showed a high prevalence of microcephaly (85%) in a sample of 60 children28. However, this high prevalence may reflect a selection bias because the hospitals in which both studies were performed were reference institutions for CZS at the time. Moreover, other systemic changes (e.g., motor and cognitive dysfunction, and other neurological disorders) were very frequently detected in our study. The high prevalence of these changes was directly related to the probable stage in which ZKV infection occurred during pregnancy. Fourteen mothers in this study reported dengue-like symptoms during the first or second trimester of pregnancy. This also led to a high prevalence of ophthalmic changes in these children.
Most eyes (37/52, 71.15%) had some abnormality. Fundus anomalies were found in 44.23% of the eyes examined and in 55.26% of the eyes of patients with microcephaly. The prevalence of ocular changes was higher in this study compared with the study conducted by Freitas et al.1, in which ocular abnormalities were found in 34.5% (10/29) of children with microcephaly. However, that study reported only fundus changes and did not focus on other common eye changes associated with CZS, such as refractive errors, eye alignment, and nystagmus. Verçosa et al.30 showed that 25 of 70 children (36%) with presumed CZS microcephaly had ocular changes. These alterations comprise fundus abnormalities, strabismus, and nystagmus; nonetheless, the study did not investigate refractive errors. Both previous studies linked microcephaly to a higher incidence of eye changes. Therefore, neurological changes have a direct relationship with the presence of ophthalmic changes, suggesting a viral infection in the gestational period as a common denominator. Therefore, it was expected that fundus abnormalities would be found in a larger proportion of children in this study who had ZKV infection during the first or second gestational trimester. However, this proportion in the present study was only 50% (7/14). This can be explained by the imprecision and subjectivity in determining the gestational age at which the infection occurred. A large number of mothers did not realize the presence of symptoms or did not show symptoms of ZKV infection; nevertheless, these mothers may have been infected with ZKV during the first two trimesters of pregnancy. In addition, confirmatory serological and/or molecular tests were not performed in most of them.
The prevalence of nystagmus and strabismus was high (19.23% and 53.85%, respectively). Verçosa et al.30 reported a prevalence of 8.57% and 14.29%, respectively. Even higher prevalence rates of strabismus have been reported in other studies (i.e., 60%17 and 92%28). These findings were common in patients with CZS, occurring as a result of both structural changes in the retina and optic nerve and neurological abnormalities.
Abnormalities of intrinsic ocular motility have rarely been described in the literature28. Ventura et al. found only one case of a defect in direct photomotor reflex among 60 children (2%)30. In the present study, four children showed decreased consensual and direct photomotor reflexes; during the evaluation, medium mydriasis and anisocoria were detected in two and one of those children, respectively. Abnormalities of the optic nerve, central neurological abnormalities, or damage to the iris muscles19 caused by the virus may be responsible for the alteration of these reflexes.
Currently, there is no known causal relationship between findings of the anterior segment (i.e., punctate keratopathy in one child and blepharitis in another) and congenital ZKV infection.
The mean SE of OU found in this study (+0.49 D) is considered normal, and was similar to those reported in other studies involving healthy children22. However, on a case-by-case basis, the prevalence of refractive errors (24 of 52 eyes [46.15%]) in patients with CZS was higher than that observed in healthy children of the same age group. In their case series, Ventura et al.17 found a high prevalence of myopia (40%), which was abnormal for the age group analyzed. In another study28, the rate of significant refractive errors, requiring glasses, was 43.7%. These findings suggest that refractive errors are common in children with CZS. Hence, these children should routinely and periodically undergo refractive evaluation.
In our sample, it was possible to infer a relationship between refractive errors and fundus abnormalities. Of the 29 eyes with normal SE, 17 had a normal fundoscopy. In contrast, of the 23 eyes with some type of ametropia, only five had a normal fundus. Similarly, patients with neurological disorders are associated with a higher incidence of refractive errors28,29. Therefore, it is crucial that children with CZS, particularly those with neurological and fundoscopic changes, receive special attention in the search for refractive errors.
Glaucoma is rarely described as a component of CZS. In this study, although some children had increased disc excavations, none of them exhibited elevations in IOP. CCT appears to be an independent variable in the development of glaucoma31,32. Of note, CCT tends to be slightly greater in children aged 2-4 years compared with adults3. The mean CCT of the children in this study was 568.33 ± 46.21 μm (range: 482.00-710.00 μm) for OU. Gul et al.4 reported similar CCT values in healthy children aged 1-2 years (i.e., 556 µm). In these children, there were no alterations related to glaucoma (e.g., APD and corneal diameter). Thus, increased papillary excavation alone, even with greater CCT, is insufficient for the diagnostic confirmation of glaucoma. Consequently, a diagnosis of glaucoma was not reached for any of our patients.
Saw et al. concluded that children with larger APD had higher weight, greater body length, or larger cranial circumference at birth27. In this study, although the mean APD of children with microcephaly was similar to that of the entire sample (20.91 ± 1.21 mm [range: 18.36-24.19 mm] vs. 21.08 ± 1.21 mm [range: 18.36-24.19 mm], respectively), the mean APD value for children without microcephaly was higher (21.55 ± 1.13 mm [range: 20.05-23.79 mm]). Therefore, there is a tendency for children with microcephaly (within the age range examined) to have eyes with smaller APDs. However, these average values are considered normal because they are similar to those reported in other studies that included children without ophthalmic or systemic changes16,24.
Although the exact ocular pathophysiology of prenatal and acquired infections is unknown, ZKV is thought to have tropism for the posterior segment of the eye, particularly the macula. Several reports on chorioretinal scars and changes in congenital retinal pigment epithelium suggested an initial change in the external retina1,28. More recently, cases of active posterior uveitis caused by ZKV in adults have been described, indicating initial inflammatory involvement in the external retina and/or the choroid25,26.
Infections with arboviruses have the potential to reach epidemic proportions. The outbreak of ZKV infection that occurred in Brazil in 2015 demonstrated the ability of viruses to spread to several regions where Aedes aegypti is endemic. Due to its devastating teratogenic effects, ZKV infection has attracted the attention of the scientific and medical community. The ophthalmological and systemic changes induced by CZS persist throughout the life of a child. Thus, it is essential to disseminate information to control the occurrence of this dreaded syndrome. Such information can include measures for the control of mosquito populations and the prevention of contagion by ZKV. Also, knowledge on the congenital abnormalities related to infection, as well as methods for the early diagnosis and treatment of these abnormalities, is important for healthcare professionals. Finally, there is a need for effective cognitive and physical rehabilitation programs targeted at patients with CZS. The above elements are crucial for reducing the personal and social consequences of CZS in children.
Limitations of the study include the small sample size used, as well as the referral of children already diagnosed with or suspected of congenital ZKV infection. Studies33 involving a larger number of children are warranted to confirm the present data.
REFERENCES
1. Freitas BP, Dias JRO, Prazeres J, Sacramento GA, Ko AI, Maia M, et al. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016;134(5):529-35.
2. Dick GW, Kitchen SF, Haddow AJ. Zika virus, I: Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509-20.
3. Besnard M, Lastère S, Teissier A, Cao-lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014,19(13):20751.
4. Center for Disease Control and Prevention (CDC). Transmission and risks.[recurso eletrônico]. Acessado em janeiro/2021. Disponível:, <http://cdc.gov/zika/transmission/index.html>
5. Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, Rosa AT, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880-2.
6. Faye O, Freire CC, Iamarino A, Faye O, Oliveira JVC, Diallo M, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8(1):e2636.
7. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536-43.
8. Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, et al. Zika virus, French Polynesia, South Pacific. 2013. Emerg Infect Dis. 2014;20(6):1085-6.
9. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedent epidemic wave os mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. 2014;19(41):20929.
10. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014; 20(10):O595-6.
11. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885-6.
12. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Monitoramento dos casos de microcefalia no Brasil até a Semana Epidemiológica 47. Informe Epidemiológico - Semana Epidemiológica 47. 2015.
13. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Protocolo de atenção à saúde e resposta à ocorrência de microcefalia relacionada à infecção pelo vírus zika. Acessado em Janeiro/2021, disponível em <https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_resposta_microcefalia_relacionada_infeccao_virus_zika.pdf>
14. Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171(3):288-95.
15. Tang H, Hammack C, Ogden SC, w z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587-90.
16. Isenberg SJ. Physical and refractive characteristics of the eye at birth and during infancy. In: Isenberg SJ, editor. The eye in infancy. 2nd edition. St. Louis: Mosby; 1994; p.36-51.
17. Ventura CV, Maia M, Ventura BV, Linden VVD, Araújo EB, Ramos RC, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79(1):1-3
18. Singh PK, Guest JM, Kanwar M, et al. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight. 2017;2(4):e92340.
19. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Secretaria de Atenção à Saúde. Orientações integradas de vigilância e atenção à saúde no âmbito da Emergência de Saúde Pública de Importância Nacional: procedimentos para o monitoramento das alterações no crescimento e desenvolvimento a partir da gestação até a primeira infância, relacionadas à infecção pelo vírus Zika e outras etiologias infeciosas dentro da capacidade operacional do SUS. Acessado em Janeiro/2022. Disponível em: <https://bvsms.saude.gov.br/bvs/publicacoes/orientacoes_integradas_vigilancia_atencao_emergencia_saude_publica.pdf>
20. Fernandez MP, Saad EP, Martinez MO, Corchuelo S, Reyes MM, Herrera MJ, et al. Ocular Histopathologic Features of Congenital Zika Syndrome. JAMA Ophthalmol. 2017;135(11):1163-9.
21. Honein MA, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59-68.
22. World Health Organization (WHO). Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero. Acessado em Janeiro/2022. Disponível em: <http://apps.who.int/iris/bitstream/10665/204475/1/WHO_ ZIKV_MOC_16.3_eng.pdf>.
23. Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001 Nov;119(11):1625-8.
24. Gul A, Caglar C, Cinal A, Yasar T, Kilic A. Ocular biometry and central corneal thickness in children: a hospital-based study. Arq Bras Oftalmol. 2014;77(3):152-4
25. Parke III DW, Almeida DR, Albini TA, Ventura CV, Berrocal AM, Mittra RA. Serologically confirmed zika-related unilateral acute maculopathy in an adult. Ophthalmology. 2016;123(11):2432-3.
26. Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. Bilateral posterior uveitis associated with Zika virus infection. Lancet. 2017;389(10064):125-26.
27. Saw SM, Tong L, Chia KS, Koh D, Lee YS, Katz J, et al. The relation between birth size and the results of refractive error and biometry measurements in children. Br J Ophthalmol. 2004;88(4):538-42.
28. Ventura LO, Lawrence L, Ventura CV, Dutton GN, Marinho P, Ferro PF, et al. Response to correction of refractive errors and hypoaccommodation in children with congenital Zika syndrome. J AAPOS. 2017;21(6):480-4.
29. McClelland JF, Parkes J, Hill N, Jackson AJ, Saunders KJ. Accommodative dysfunction in children with cerebral palsy: a population-based study. Invest Ophthalmol Vis Sci. 2006;47(5):1824-30.
30. Verçosa I, Carneiro P, Verçosa R, Girão R, Ribeiro EM, Pessoa A, et al. The visual system in infants with microcephaly related to presumed congenital Zika syndrome. J AAPOS. 2017;21(4):300-304.
31. Amini H, Fakhraie G, Abolmaali S, Amini N, Daneshvar R. Central Corneal Thickness in Iranian Congenital Glaucoma Patients. Middle East Afr J Ophthalmol. 2012;19(2):194-8.
32. Gordon MO, Beiser JA, Kass MA. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20; discussion 829-3.
33. Ferreira CCM, Tavares IM. Intraocular pressure and central corneal thickness in full-term newborns. Arq Bras Oftalmol. 2017;80(5):313-6.



Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
July 21, 2022.
Accepted on:
December 17, 2022.