Francyne Veiga Reis Cyrino1; Breno Reis Almeida2
DOI: 10.17545/eoftalmo/2019.0003
ABSTRACT
Myopia is one of the most common refractive errors whose prevalence has been reported to be increasing worldwide. Myopic choroidal neovascular membrane occurs in 5%–11% of pathological myopia cases. The aim of this study was to evaluate the response of myopic choroidal neovascular membrane (CNVM) treated with either bevacizumab or ranibizumab and to analyze the results. This study was a retrospective analysis of 28 eyes of 28 patients with myopic CNVM treated with anti-VEGF therapy (bevacizumab or ranibizumab. No correlation was observed between age and degree of myopia. Ranibizumab and bevacizumab demonstrated similar therapeutic effects in the treatment of myopic membranes with fewer injections unlike age-related macular degeneration. There could be a relationship between the onset of symptoms, treatment, and VA improvement. Efforts are needed to start anti-VEGF therapy as earlier as possible.
Keywords: Myopia; Bevacizumab; Ranibizumab.
RESUMO
A miopia é uma das ametropias mais comuns e sua prevalência está aumentando em todo o mundo, segundo publicações recentes. A membrana neovascular coroidal (MNVC) miópica ocorre em 5%–11% dos casos patológicos de miopia. O objetivo deste estudo foi avaliar a resposta da MNVC miópica ao tratamento com bevacizumabe ou ranibizumabe e analisar os resultados. O estudo consistiu em uma análise retrospectiva de 28 olhos de 28 pacientes com MNVC miópica tratados com um antagonista do fator de crescimento endotelial vascular, bevacizumabe ou ranibizumabe. Não houve recidiva após o tratamento e em 22 olhos, houve ganho de quatro ou mais linhas de visão. Não observamos correlação entre o surgimento da MNVC miópica, a idade e o grau de miopia. Tanto o ranibizumabe quanto o bevacizumabe demonstraram efeitos terapêuticos semelhantes no tratamento da MNVC miópica, sendo necessários um menor número de injeções em relação a degeneração macular relacionada à idade. É possível que haja uma relação entre o aparecimento dos sintomas, o tempo decorrido até o início do tratamento e a melhora da acuidade visual e sugerimos que se deva iniciar a terapia anti-VEGF o mais precocemente possível.
Palavras-chave: Miopia; Bevacizumab; Ranibizumab.
RESUMEN
La miopía es una de las ametropías más comunes y su prevalencia se está incrementando en todo el mundo, según publicaciones recientes. La membrana neovascular coroidea (MNVC) miópica ocurre en un 5%-11% de los casos patológicos de miopía. El objetivo de este estudio fue evaluar la respuesta de la MNVC miópica al tratamiento con bevacizumab o ranibizumab y analizar los resultados. El estudio consistió en un análisis retrospectivo de 28 ojos de 28 pacientes con MNVC miópica tratados con un antagonista del factor de crecimiento endotelial vascular, bevacizumab o ranibizumab. No hubo recidiva después del tratamiento y en 22 ojos hubo aumento de cuatro o más líneas de visión. No observamos correlación entre el surgimiento de la MNVC miópica, la edad y el grado de miopía. Tanto el ranibizumab como el bevacizumab demostraron efectos terapéuticos semejantes en el tratamiento de la MNVC miópica, siendo necesarios un menor número de inyecciones con relación a la degeneración macular relacionada a la edad. Es posible que haya una relación entre el aparecimiento de los síntomas, el tiempo transcurrido hasta el inicio del tratamiento y la mejora de la acuidad visual, y sugerimos que se deba iniciar la terapia anti-VEGF lo antes posible.
Palabras-clave: Miopía; Bevacizumab; Ranibizumab.
INTRODUCTION
Myopia is one of the most common refractive errors affecting approximately 24% of adults aged >40 years. Recent studies have reported a global increase in the prevalence of myopia, especially in Europe and East Asia1-5, where 90% of the teenagers are affected, probably due to indoor activities and the use of electronic devices such as cell phones and computers6.
Choroidal neovascular membrane (CNVM) is a serious complication that occurs in approximately 5%–11% of pathological myopia (PM) cases7, although it can occur in any degree of myopia with or without degenerative fundus changes8. After age-related macular degeneration (AMD), PM is the second most common cause of choroidal neovascularization and affects approximately 62% of myopic patients aged <50 years9. It generally occurs in the subfoveal space, leading to metamorphopsia, significant visual loss, and legal blindness if left untreated10.
Recently, anti-vascular endothelial growth factor has been confirmed to be the first-line therapy for choroidal neovascularization secondary to AMD, and numerous studies evaluating CNVMs caused due to PM have also reported its benefits. This study was conducted to evaluate the response of myopic CNVM to treatment with either bevacizumab or ranibizumab and to analyze the results.
METHODS
This study was a retrospective analysis of 28 eyes of 28 patients diagnosed with myopic CNVM and treated with anti-VEGF therapy (bevacizumab and ranibizumab) between February 2014 and July 2017, with at least 6 months of follow-up. Patients with diabetes, AMD, inflammatory CNVM, or idiopathic CNVM were excluded from this study.
All patients underwent a complete ophthalmic evaluation with the best corrected visual acuity (BCVA) at initial presentation, and at 6 months, the degree of myopia, fluorescein angiography patterns (AF), SD-OCT images (before and after treatment), and the mean number of anti-VEGF injections required for CNVM scarring were evaluated. Intravitreal anti-VEGF injections were administered between 5 and 10 days after diagnosis. The choice of the antiangiogenic drug was based on the patient’s financial commitment or the contractual coverage of the insurance.
After the initiation of treatment, monthly follow-up was performed in the first 3 months after intravitreal injections and at 6 months. Reinjection was performed pro re nata (PRN) if the patient was symptomatic and/or if there was a change in the SD-OCT images. BCVA changes by more than two lines or less than two lines on the Snellen chart were considered respectively as improvement or worsening.
RESULTS
A total of 28 eyes (4 males and 24 females) were included in this study, with the number of females being predominant (86%). Age varied between 25 and 78 years (mean age 41.4 years), and the degree of myopia ranged from −3.00 to −16.50 D (mean −7.54D). Three patients displayed a macular scar in the contralateral eye with history of no previous treatment of CNVM and visual acuity from 20/200 to counting fingers. The duration of onset of symptoms varied from 7 days to 3 months (mean 3.5 weeks), and the BCVA before the treatment ranged from 20/40 to 20/200 (mean BCVA 20/100). SD-OCT (Fig. 1) and AF were performed in all patients, and AF demonstrated classic myopic CNVM in all eyes.
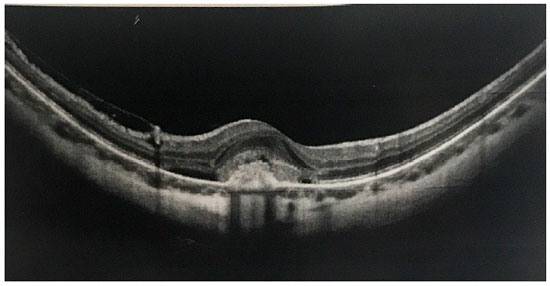
Anti-VEGF intravitreal injections were administered at the beginning of and after the treatment on a PRN basis. A total of 16 eyes were treated with intravitreal bevacizumab (1.25 mg in 0.5 ml), and 12 eyes were treated with ranibizumab (0.5 mg in 0.5 ml). The mean number of injections was 1.24 (range 1–5). Juxtafoveal CNVM was observed in 17 eyes (60.71%), subfoveal CNVM was observed in 11 eyes (39.28%), and none had extra-foveal membrane (Fig. 2).
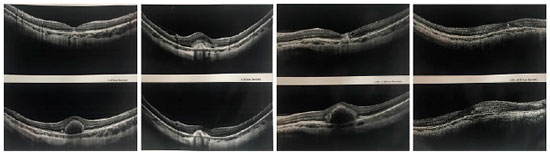
A total of 20 eyes (71.42%) demonstrated CNVM regression with a single injection (either bevacizumab or ranibizumab), 1 eye required three injections of bevacizumab, and two eyes required three injections of ranibizumab to stabilize. Only one eye required more than three injections of ranibizumab (Fig. 3). After the completion of the treatment, all myopic CNVMs remained inactive and did not recur during the follow-up period (6 months, 1–3 years) (Fig. 2).
Regarding visual outcome, two eyes remained stable (no vision improvement: 7.14%), 4 eyes have won three lines of Snellen (14.28%), and 22 eyes had improved vision in four or more lines (78.58%). No difference was observed between ranibizumab and bevacizumab in terms of visual outcome on the Snellen chart.
DISCUSSION
Myopic choroidal neovascularization is generally of classic type two with small membranes that may show a small amount of liquid, no drusen, and a small hemorrhage, as observed in this study as well. Contralateral involvement occurs in one-third of patients within 8 years and has poor prognosis if left untreated11. In Japan, myopic CNVM is the leading cause of unilateral or bilateral blindness, whereas in China, it is the major cause of blindness among people aged >40 years. There is a lack of studies on the prevalence of myopic membranes in Brazil; however, in this study, none of the patients developed CNVM in the fellow eye during the 3 years of follow-up, which is in contrast to the findings of Leveziel et al12.
We also observed a higher incidence of juxtafoveal CNVM (60.71%), which is consistent to the findings already described by Yodoi et al. (54.5%). Only one case of CNVM occurred at the extrafoveal region13. No relationship was observed between age, degree of myopia, number of injections, and visual acuity gain as previously reported in the literature5,8,12.
However, we did observe instead a correlation between the onset of symptoms, beginning of the treatment, and visual acuity improvement. Patients who were treated as soon as they noticed the visual loss had better results than those treated later (>1 month) and required fewer injections. In this study, visual acuity was improved in 92.86% of treated eyes.
Treatment of myopic choroidal neovascularization involves photodynamic therapy (PDT) or anti-VEGF injections. Retrospective studies have demonstrated that PDT may lead to worsening of visual acuity after 3 and 7 years of treatment secondary to pigment epithelium atrophy14-16. Results of major randomized clinical trials indicate that antiangiogenic therapy requires much fewer injections to treat myopic CNVM than AMD, a result that was similar to ours.
CONCLUSION
Ranibizumab and bevacizumab demonstrated similar therapeutic and safety effects in the treatment of myopic membranes despite having small variations in their formula. Unlike AMD, the number of injections required is limited, reducing the incidence of complications along with significant improvement of visual acuity. Efforts are needed to start anti-VEGF therapy as earlier as possible, because of the relationship between the onset of symptoms, treatment, and visual acuity.
REFERENCES
1. Avila MP, Weiter RJJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984; 91:1573-1581.
2. Hammond CJ. Myopia prevalence in Europe: cohort effect of increasing prevalence not fully explained by higher educational levels. Orlando, Florida: The Association for Research in Vision and Ophthalmology 2014 Annual Meeting. 2014 May 4 and 8.
3. Wu HM, Seet B, Yap EP, et al. Does education explain ethnic differences in myopia prevalence? A population-based study of young adult males in Singapore. Optom Vis Sci. 2001; 78: 234-239.
4. Feisal AA, Luong M, Munro M, Tufail A. The other CNVM: A review of myopic choroidal neovascularization treatment in the age of anti-vascular endothelial growth factor agents. Survey of Ophthalmol. 2015; 60(3):204-215.
5. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016; 52:156-187.
6. Dolgie E. The myopia boom. Nature. 2015; 519:276-278.
7. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidencebased systematic review. Am J Ophthalmol. 2014; 157:9-25.e12.
8. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003; 87:570-573.
9. Neelam K, Cheung CM, Ohno-Matsui K, et al. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012; 31:495-525.
10. Silva R. Myopic maculopathy: a review. Ophthalmologica. 2012; 228:197-213.
11. Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003; 87(5):570-573.
12. Leveziel N, Caillaux V, Bastuji-Garin S, Zmuda M, Souied EH. Angiographic and optical coherence tomography characteristics of recent myopic choroidal neovascularization. Am J Ophthalmol. 2013; 155:913-919.
13. Yodoi Y, Tsujikawa A, Nakanishi H, Otani A, Tamura H, Ojima Y, et al. Central retinal sensitivity after intravitreal injection of bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2009; 147:816-824.
14. Ng DS, Kwok AK, Chan CW. Anti-vascular endothelial growth factor for myopic choroidal neovascularization. Clin Exp Ophthalmol. 2012; 40(1):98-110.
15. Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, Wong TY, Silva R, Pilz S, Gekkieva M; RADIANCE Study Group. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014; 121(3):682-692.
16. Chang LK, Spaide RF, Brue C, Freund KB, Klancnik JM Jr, Slakter JS. Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age related macular degeneration. Arch Ophthalmol. 2008; 126:951-954.


Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: The authors have no potential conflicts of interest to disclose
Received on:
October 11, 2018.
Accepted on:
February 1, 2019.