Rubens Belfort Mattos Neto
DOI: 10.17545/e-oftalmo.cbo/2015.5
ABSTRACT
Melanoma is the most common primary intraocular cancer. Relatively rare, choroidal melanoma is a very important cause for vision loss and death in Brazil, probably due to late diagnosis. The purpose of this article is a brief review of choroidal melanoma with particular focus on clinical pictures.
Keywords: Choroid Neoplasms. Choroid. Uvea.
RESUMO
OBJETIVO: O melanoma de coroide é o câncer primário intraocular mais comum em adultos. Apesar de relativamente raro segue como causa comum de perda de visão e causa de morte no Brasil, principalmente pelo diagnóstico tardio desse tumor. Este artigo tem como objetivo uma breve revisão das principais características dessa doença, com enfoque em imagens clínicas.
Palavras-chave: Neoplasias da Coroide. Coroide. Úvea.
INTRODUCTION
Melanoma can affect different parts of the eye, from the eyelids and conjunctiva to internal structures such as the iris, ciliary body, and choroid. Skin and conjunctiva melanomas should be considered as different diseases from intraocular melanomas because they do not share risk factors and disease behavior. Ultraviolet exposure, for example, is an important risk factor for skin melanoma but not for choroidal melanoma.1,2
Choroidal melanoma is the most common primary intraocular cancer in adults, with an annual incidence of approximately six cases per million individuals. In addition, it is the most common cancer in the uvea. Here we review this type of cancer, focusing on clinical images.
RISK FACTORS
Caucasians with blond or red hair and light eyes have an augmented risk of developing choroidal melanoma. Advanced age and the presence of choroidal nevus or oculodermal melanocytosis (nevus of Ota) are also risk factors.3 Although light hair and eyes are risk factors, it is noticeable that in Brazil, there are melanoma patients with dark hair and pigmented or even black skin.
Among these, the presence nevus and oculodermal melanocytosis is the most important risk factor when considering patient follow-up and early melanoma diagnosis.
It is thought that environmental factors such as sun exposure are not as important risk factors for the onset of intraocular melanoma as they are for the onset of skin melanoma. Therefore, there is no indication of the use of sunglasses for the prevention of choroid melanoma.4
a) Choroidal Nevus
Choroid nevi are benign hamartomas present in part of the population. They are usually asymptomatic but can also be associated with vision loss and retinal abnormalities. Anatomically, the lesion is flat, thinner than 1 mm, and has a base smaller than 5 mm.5
Congenital nevi are present in approximately 5% of the population. Rarely, these lesions may undergo malignant transformation and develop into choroidal melanoma.6
Secondary retinal abnormalities associated with lesion growth were identified as risk factors for nevus growth. These risk factors include subretinal fluid, symptoms, orange pigment (lipofuscin), optical disk proximity, thickness greater than 2 mm, anechoic ultrasound images, and the absence of a hypopigmented lesion halo (Figure 1).7
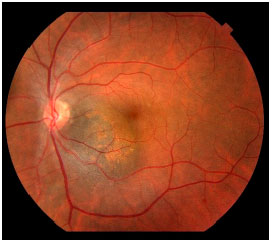
These risk factors are not sufficient for the diagnosis of melanoma; however, they indicate increased risk of growth. In some cases, the ophthalmologist may prescribe nevus treatment on the basis of this risk.
The annual risk of malignant transformation of common nevus was estimated to be approximately one in 8845 individuals in the American Caucasian population.6 It is important to emphasize that the vast majority of nevus will never develop into choroidal melanoma.
The presence of nevus with no associated risk factors should be followed up annually and, if possible, photographically documented at the onset. Nevus with associated risk factors should be closely monitored every 3 months in the first year and then every 6 months. In such cases, in addition to photography, other diagnostic methods such as ultrasound and optical coherence tomography (OCT) may be useful. If lesion growth is observed, malignant transformation should be considered and proper treatment should be provided.
Lesions thicker than 3 mm are diagnosed as melanoma, while lesions between 1 and 3 mm can be diagnosed as large nevi and minor melanomas. In such cases, risk factors are very important for diagnosis. Documented growth indicates that the lesion is a choroidal melanoma until proven otherwise.
b) Oculodermal Melanocytosis
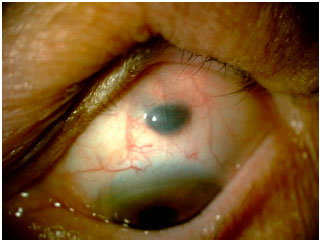
Oculodermal melanocytosis is a congenital disease characterized by increased pigmentation in the ocular tissues, with or without associated skin hyperpigmentation in the trigeminal region. When such skin hyperpigmentation is present, the disease is also known as nevus of Ota. Nowadays, the use of the term ocular or oculodermal melanocytosis is preferred, depending on the affected structures (Figure 2).
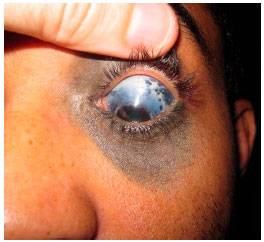
In the eye, melanocytosis is observed as increased pigmentation of the sclera but not of the conjunctiva. Moving the conjunctiva with a cotton swab can help differentiate between the pigmentation of these structures. Because the pigmentation is in the sclera, the pigment should not move with the conjunctiva in this test. In addition to the sclera, uveal hyperpigmentation is usually present. Hyperpigmentation can also affect the ocular orbit and meninges.
Ocular melanocytosis increases the risk of choroidal melanoma, which is 1:400 in these patients. These patients are also at an increased risk of glaucoma. Therefore, intraocular pressure, besides eye fundus, should be examined every 2 years.3
CHOROIDAL MELANOMA
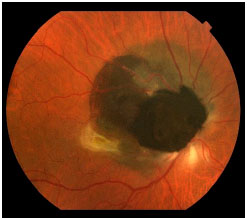
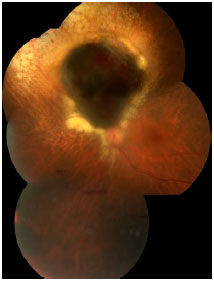
Choroidal melanoma is a subretinal lesion, usually single, well defined, and pigmented (Figure 3).8 Usually, it is not associated with extensive retinal detachment or vitreous hemorrhage, with exceptions (Figure 4).
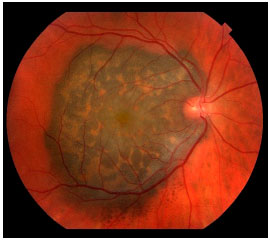
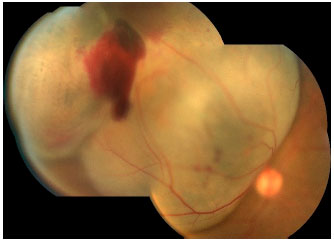
SYMPTOMS
Symptoms are variable and depend on the tumor size and location within the eye. If the tumor is close to the macula, the patients usually report visual blurring or partial visual loss (Figure 5). However, if the tumor is in the periphery, it can considerably grow before the patient notices any symptoms.
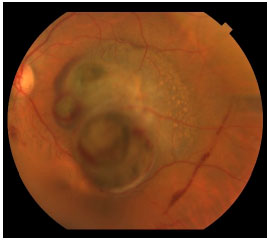
Often, the tumor is asymptomatic and discovered during a routine or preoperative cataract surgery examination. Unfortunately, in Brazil, large tumors are usually diagnosed. This is because there is no access to adequate health services that could provide early diagnosis (Figure 6). Eye fundus examination with dilated pupils is fundamental for any patient with visual loss complaints. Symptoms include floaters, photopsia, and blurring or reduced visual acuity.
DIAGNOSIS
The most important test for the diagnosis of choroidal melanoma is fundus examination by indirect ophthalmoscopy with dilated pupils. The examination allows the ophthalmologist to identify the subretinal lesion (pigmented or not).
Ocular ultrasound, associated with fundus examination, is necessary for the diagnosis of choroidal melanoma. These two tests enable a correct diagnosis in 90% of cases. Accurate diagnosis also depends on the correct identification of the lesion morphology, size, and reflectivity by the ophthalmologist during ultrasound examination.
a) Lesion Morphology
B mode allows to determine the lesion shape, which is usually in dome or mushroom. The lesion progressively grows and undergoes resistance by Bruch’s membrane. When Bruch’s membrane ruptures, the lesion may grow with less resistance, creating a mushroom appearance, very characteristic of choroidal melanoma, although not pathognomonic.
b) Lesion Size
Lesion size that measures in millimeters enables the differentiation between melanomas and nevus, besides the determination of proper treatment (enucleation or brachytherapy). These measures also enable the tracking of suspicious lesions because their growth indicates the presence of melanoma.
c) Lesion Internal Reflectivity
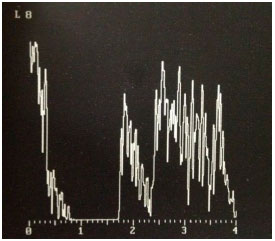
Reflectivity measurement during ultrasound mode A helps in the confirmation of diagnosis. Choroidal melanoma has medium to low reflectivity, with higher reflectivity in the lesion apex and progressive reduction associated with the formation of the famous Kappa angle (Figure 7). Ultrasound also enables the observation of choroidal excavation and lesion internal vascularity, which are suggestive of choroidal melanoma.9
Other tests such as OCT and fluorescent angiography may be necessary in selected cases.
In cases with pigmented lesions with clinical and sonographic melanoma features, there is usually no diagnostic uncertainty. In cases with amelanotic lesions, it is important to perform differential diagnosis for metastatic and inflammatory lesions. As a rule, métastasés may be multiple or affect both eyes, are associated with retinal detachment, and exhibit high reflectivity in ultrasound examination. In such cases, primary cancer diagnosis should be considered because 43% of patients have no history of cancer when they present with ocular metastasis. Cancers that most commonly progress to show ocular metastasis include breast, lung, gastrointestinal, kidney, skin, and prostate cancers.10 If a primary cancer is identified, it should be considered a metastasis until proven otherwise. Intraocular biopsy should be considered in cases where no primary lesion is identified and where ocular lesions that are very suggestive of metastasis are present.
DIFFERENTIAL DIAGNOSIS
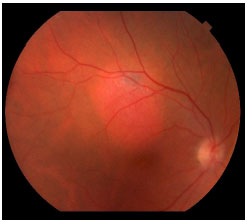
The most common differential diagnoses of choroidal melanoma include choroid nevus, EPR congenital hypertrophy, peripheral exudative hemorrhagic chorioretinopathy, hemorrhagic EPR detachment, circumscribed choroidal hemangioma (Figure 8), melanocytomas (Figure 9), age-related macular degeneration, and others.8
STAGING AND METASTASIS DISEASE
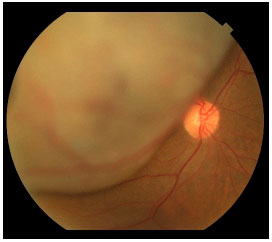
Choroidal melanoma can lead to metastasis via blood vessels, usually in the liver. The intraocular content has no lymphatic drainage; therefore, lymph node involvement is not observed, except in cases with extraocular melanoma extension (Figure 10). It has been shown that from the time of diagnosis, malignant cells may be circulating in the peripheral blood and that all patients have these cells.11,12 In contrast, only some patients develop metastatic disease, which can occur up to a decade after primary tumor treatment. The cause of this observation remains unclear.
Unfortunately, metastatic disease usually causes death in 5 months, regardless of the treatment.13 The only method to decrease the mortality rate in these patients is to perform early diagnosis and appropriate treatment. Larger lesions have an increased chance of metastasis, and despite the fact that their treatment does not change the natural history of the disease, if the injury is not treated and the lesion grows, the chance of death increases. In addition, if inappropriate treatment is provided and the lesion grows, there is an increased chance of metastasis and death.
At diagnosis, the patient is staged considering that the primary site of metastasis is the liver, followed by the lungs. In most cases, the patient does not have métastasés at the diagnosis of ocular lesions.13 These patients are evaluated through image techniques, which usually include abdomen USG or computed tomography (CT) scan, and blood tests (TGO, TGP, alkaline phosphatase, DHL, and gamma-GT).
Commonly, the presence of other tumors is assessed by X-ray or chest and pelvis CT and mammography for women. The tests are usually repeated every 3 months in the first year and every 6 months for 5 years after the treatment. The ophthalmologist may request these tests and directs the patient to the oncology or hepatology medical specialist in case of abnormal results.
At present, there are genetic tests that can be performed on the tumor tissue removed after enucleation or biopsy in tumors treated with brachytherapy.14 These genetic tests help determine the risk of metastasis and prognosis; however, because there is no known treatment for patients with a higher risk of metastasis, these tests remain controversial.
TREATMENT
The first goal of the treatment of choroidal melanoma is to save the patient’s life, and such treatment depends on the tumor size. Choroidal melanoma is not sensitive to chemotherapy and is barely sensitive to radiotherapy; therefore, systemic chemotherapy and external beam radiotherapy are not efficient treatments.
Although many treatments are described for this tumor, including laser treatment and resection surgery, enucleation and brachytherapy are still considered the gold standard strategy.
a) Enucleation
In enucleation surgery, the affected eye is removed, allowing definitive treatment of ocular lesions with a minimal risk and cost. Is indicated for large tumors (thicker than 9 mm) or when brachytherapy is not available. A sclera-coated Mules sphere may be used, besides the traditional polyethylene implants, which are often very expensive in Brazil. Implant placement during surgery provides excellent cosmetic results, with subsequent prosthesis adaptation (usually for 6 weeks after surgery).
As opposed to brachyterapy, after enucleation, it is not necessary for the patient to frequently return to a complex medical center to see an ocular oncologist. Therefore, this approach should be considered for patients who cannot or will not move to such centers for follow-ups.
The removed eye should be sent for anatomical and pathological evaluation, which confirms the diagnosis and provides additional information about the tumor, in addition to prognostic factors.
b) Brachytherapy
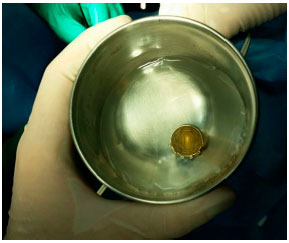
Brachytherapy consists of surgical placement of a small radioactive implant (Figure 11) outside the eye and adjacent to the sclera (Figure 12).15 The brachytherapy plate is sutured to the sclera after its correct location is determined by indirect ophthalmoscopy or transillumination. We recommend the use of intraoperative ultrasound to confirm the correct location of the plaque (Figure 13). The duration of treatment depends on the thickness of the tumor and the radioactivity of the plate, until the tumor receives 85 Gy at its apex. After the treatment, the plaque is surgically removed.16
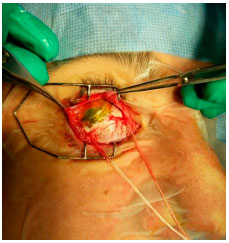
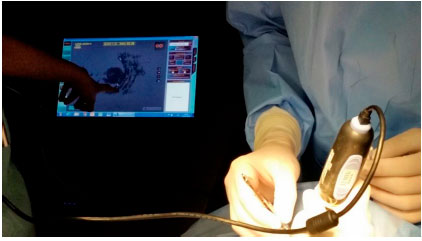
Brachytherapy is often very effective in controlling choroidal melanoma, with 95% of the lesions being properly treated (Figure 14). The disadvantages of this technique are its high cost and limited availability in the Brazilian public healthcare system. The major complication of brachytherapy is impaired vision because of retinal and optic nerve irradiation, which affects approximately 60% of patients after 2 years. Other complications are less frequent.
Patients who underwent brachytherapy are monitored by ophthalmological examination and ocular ultrasonography to confirm the tumor’s response to the treatment. The frequency of follow-up frequency varies; however, it is usually performed every 3 or 6 months.
OTHER TREATMENTS
Endoresection is a new experimental technique in which the tumor is removed through vitrectomy, preserving the eye and possibly preserving the patients’ vision. This technique is being evaluated in medical centers in Brazil, Europe, and the Middle East.
Laser therapy for the treatment of choroidal melanomas has been considered to be a therapeutic modality, particularly with transpupillary thermotherapy (I I I). Today, at present, TTT is thought to be an unsafe method for tumor treatment.17 Possibly, the good results observed were based on the treatment of small lesions that could be atypical nevi instead of small melanomas.
Scientific studies suggest that there is no difference in the mortality rates of patients who underwent brachytherapy and enucleation.18
Treatment seeks to control the tumor and decrease metastasis risks, particularly considering that métastasés are not treatable in most cases. Obviously, vision and eye preservation are also considered when managing these tumors; however, saving the patient’s life is always the major concern.
FOLLOW-UP
Ophthalmologists expert in tumors monitor patients with ocular melanoma. It is known that the risk of metastasis is increased in the first 5 years after treatment. In recent years, the treatment of skin melanoma has improved, and it is expected that soon, the treatment of choroidal melanoma métastasés will also improve, with increased patient survival.
REFERÊNCIAS
1 Schwartz LH, Ferrand R, Boelle PY, Maylin C, D’Hermies F, Virmont J. Lack of correlation between the location of choroidal melanoma and ultraviolet-radiation dose distribution. Radiat Res. 1997;147(4):451-456. http://dx.doi.org/10.2307/3579502.
2 Moan J, Grigalavicius M, Baturaite Z, Dahlback A, Juzeniene A. The relationship between UV exposure and incidence of skin cancer. Photodermatol Photoimmunol Photomed. 2015;31(1):26-35. http://dx.doi.org/10.1111/phpp.12139.
3 Shields CL, Kaliki S, Livesey M, Walker B, Garoon R, Bucci M, Feinstein E, Pesch A, Gonzalez C, Lally SE, Mashayekhi A, Shields JA. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013;131(8):993-1003. http://dx.doi.org/10.1001/iamaophthalmol.2013.129.
4 Pane AR, Hirst LW. Ultraviolet light exposure as a risk factor for ocular melanoma in Queensland, Australia. Ophthalmic Epidemiol. 2000;7(3):159-167. http://dx.doi.org/10.1076/0928-6586(200009)731-VFT159.
5 Singh AD, Belfort RN, Sayanagi K, Kaiser PK. Fourier domain optical coherence tomographic and auto-fluorescence findings in indeterminate choroidal melanocytic lesions. BrJ Ophthalmol. 2010;94(4):474-478. http://dx.doi.org/10.1136/bjo.2009.162636.
6 Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112(10): 1784-1789. http://dx.doi.org/10.1016/j.ophtha.2005.06.011.
7 Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Mashayekhi A, Shields JA. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127(8): 981-987. http://dx.doi.org/10.1001/archophthalmol.2009.151.
8 Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014;25(3):177-185. http://dx.doi.org/10.1097/ICU.0000000000000041.
9 Rochéis R. [The origin of the choroidal excavation in b-scan-sonography--an experimental and clinical study (author’s transi)].” Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981 ;217(3):193-197. http://dx.doi.org/10.1007/BF00411150.
10 Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal métastasés. Ophthalmology. 1997;104(8):1265-1276. http://dx.doi.org/10.1016/S0161-6420(97)30148-1.
11 Callejo SA, Antecka E, Blanco PL, Edelstein C, Burnier Jr MN. Identification of circulating malignant cells and its correlation with prognostic factors and treatment in uveal melanoma. A prospective longitudinal study. Eye (Lond). 2007; 21(6):752-759. http://dx.doi.org/10.1038/sj.eye.6702322.
12 Fernandes BF, Belfort RN, Di Cesare S, Burnier Jr MN. Circulating uveal melanoma cells: should we test for them? Can J Ophthalmol. 2008;43(2):155-158. http://dx.doi.org/10.3129/i08-011.
13 Cerbone L, Van Ginderdeuren R, Van den Oord J, Fieuws S, Spileers W, Van Eenoo L, Wozniak A, Sternberg ON, Schoffski P. Clinical presentation, pathological features and natural course of metastatic uveal melanoma, an orphan and commonly fatal disease. Oncology. 2014;86(3):185-189. http://dx.doi.org/10.1159/000358729.
14 Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, et al. Collaborative Ocular Oncology Group report number 1 : prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596-1603. http://dx.doi.org/10.1016/j-ophtha.2012.02.017.
15 Giblin ME, Shields JA, Augsburger JJ, Brady LW. Episcleral plaque radiotherapy for uveal melanoma. Aust N Z J Ophthalmol.1989;17(2):153-156. http://dx.doi.org/10.1111/j.1442-9071.1989.tb00505.x.
16 Shields JA, Shields CL. Management of posterior uveal melanoma: past, present, and future: the 2014 Charles L. Schepens lecture. Ophthalmology. 2015;122(2):414-428. http://dx.doi.org/10.1016/j.ophtha.2014.08.046.
17 Mashayekhi A, Shields CL, Rishi P, Atalay HT, Pellegrini M, McLaughlin JP, et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: importance of risk factors in tumor control. Ophthalmology. Forthcoming, 2014. http://dx.doi.org/10.1016/j-ophtha.2014.09.029.
18 Margo CE. The collaborative ocular melanoma study: an overview. Cancer Control. 2004;11(5):304-309.

Funding sources: none declared.
Conflict of interest: none declared.
Received on:
January 15, 2015.
Accepted on:
January 30, 2015.