Marcos Ávila1; José Maurício Botto de B. Garcia2; David Leonardo Cruvinel Isaac3
DOI: 10.17545/e-oftalmo.cbo/2015.18
ABSTRACT
OBJECTIVE: The present study aimed to discuss the current management for dry and wet age-related macular degeneration (AMD). Emerging diagnostic and therapeutic options supported by the results of pivotal studies, different approaches such as “pro re nata (PRN)” and “treat-and-extend” protocols, and future trends have reshaped the AMD-treatment paradigm.
METHODS: This descriptive report provides a comprehensive analysis, focusing on the present “state of the art” literature, and also highlights some plausible pathways for future AMD treatment strategies.
RESULTS: The current understanding of the different angiogenesis and inflammatory mediators, including vascular endothelial growth factor, platelet-derived growth factor, and placental growth factor, has changed our understanding about AMD pathogenesis. This data has altered treatment options, aiming optimal results. Most of these pathways are still in the experimental stage, and may represent future alternatives, considering the chronic description of AMD. The antiangiogenic drugs remain the mainstay of AMD treatment, but recent trials, such as combination therapy, have revealed promising results. Optical coherence tomography advances also deserve special attention, since it is an important tool that is used on a regular basis for management of this condition.
CONCLUSION: In spite of the significant advances in dry and wet AMD treatments, patients still require frequent injections and clinical appointments. Future trends have the potential to change this paradigm. AMD is a chronic disorder, and the consensus is that the best results will be accomplished with prompt detection and treatment, independent of the treatment strategy selected.
Keywords: Macular Degeneretion. Angiogenesis Inhibitors. Neovascularization. Pathologic. Tomography. Optical Coherence.
RESUMO
OBJETIVO: O objetivo deste estudo é discutir o manejo atual da degeneração macular relacionada à idade (DMRI), seca e úmida. Novas opções diagnosticas e terapêuticas, baseadas em resultados de estudos importantes, com diferentes abordagens como os protocolos “PRN” e “tratar e estender” e em tendências futuras, têm remodelado o paradigma do tratamento da DMRI.
MÉTODOS: Este estudo descritivo apresenta uma análise abrangente, focada em informações atuais do “estado da arte”, além de esclarecer algumas trajetórias futuras plausíveis de abordagem da DMRI.
RESULTADOS: O conhecimento atual sobre distintos mediadores “angiogênicos” e inflamatorios, incluindo VEGFs, FCDP e PIGF entre outros, modificou a compreensão sobre a patogênese da DMRI. Esses dados alteraram as opções de tratamento objetivando otimizar os resultados. Considerando o caráter crônico da DMRI, a maioria dessas opções, ainda em modelos experimentais, pode representar alternativas futuras. As drogas antiangiogênicas ainda constituem a linha principal de tratamento, porém ensaios recentes, tais como a terapia combinada têm demonstrado resultados promissores. Os avanços da TCO merecem atenção especial, por se tratar de uma ferramenta importante para condutas rotineiras.
CONCLUSÃO: Apesar de avanços notáveis no tratamento da DMRI seca e úmida, os pacientes ainda necessitam frequentemente de injeções e consultas clínicas. As tendências futuras podem potencialmente mudar esse paradigma. A DMRI é um distúrbio crônico e, independentemente da estratégia de tratamento utilizada, existe um consenso de que os melhores desfechos são alcançados com detecção e tratamento precoces.
Palavras-chave: DRMI. Degeneração Macular. Inibidores da Angiogénese. Neovascularização Patológica. Tomografia de coerência óptica.
INTRODUCTION
Age-related macular degeneration (AMD) is the most common cause of blindness in the developed world. This disease accounts for >50% of all cases of legal blindness in the United States, and its incidence is expected to double in 2020. AMD has been classically divided into two forms-a dry or atrophic form, and a wet form (also called neovascular AMD)-illustrated by the development of choroidal neovascularization (CNV).
Although approximately 80% of patients have the dry form, 90% of the cases of blindness from this disease have wet AMD2. Geographic atrophy (GA) represents the most advanced stage of the dry form. Treatment options are only available for the wet form of the disease in the form of the antiangiogenic agents which prevent further deterioration of visual function and provide some improvement. Currently, there are no treatments for the dry form.
Both heritable and environmental risks have been implicated in the development of AMD. Smoking is the main modifiable risk factor and has been consistently identified in various reports2. The Age-Related Eye Disease Study (AREDS) trials demonstrated that daily oral supplementation with antioxidant vitamins and minerals reduced the possibility of emergent advanced AMD by 25% in a selected group of patients at 5 years. Genetic evaluations have identified 19 susceptibility loci that seem to be associated with >50% of AMD progression risk; these include single polymorphisms in complement factor H (CFH) and in age-related maculopathy susceptibility 2 (ARMS2). Awh et al suggested that the administration of antioxidant supplements should be modified in certain subgroups of patients based on their CFH and ARMS2 genotypes; however, this hypothesis has not been confirmed.
It is critical to detect CNV before substantial damage occurs in order to maximize successful outcomes after antiangiogenic therapy. Lim et al showed that delayed intervention lead to insufficient treatment, irreversible macular damage, and poorer outcomes. In this study, a delay of 14 weeks in the diagnosis doubled the probability for visual acuity worsening after treatment7.
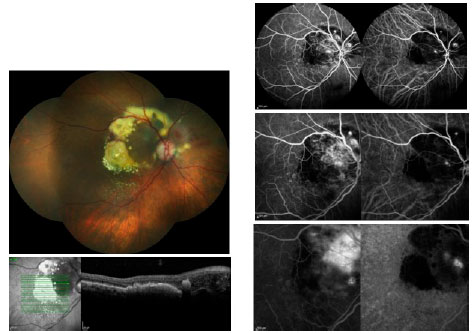
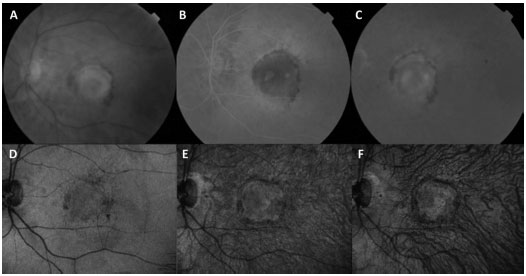
During the initial consult for a patient with suspected AMD, the performance of a fluorescein angiography (FA) is required when it is not contraindicated. This test helps with the diagnosis and treatment planning and may detect various forms of wet AMD when used in conjunction with indocyanine green angiography (ICGA). Retinal angiomatous proliferation (RAP), also called CNV type 3, is clinically characterized by focal bleeding, cystic abnormalities, and lipid exudates within the retina. Polypoidal choroidal vasculopathy (PCV) is difficult to distinguish clinically from other forms of occult CNV (CNV type 1), and often presents with recurrent pigment epithelium detachments (PEDs)8. The FA for PCV displays a similar occult pattern, and the ICGA is able to define the polypoidal alterations in distinct detail (Figure 1).
Optical coherence tomography (OCT), first used in the 1990s, is arguably the most frequently used tool in the long-term management of wet AMD. The comparisons of macular thickness and morphology over time allow assessment of the treatment response.
In this report, we will discuss the current management of AMD, including diagnostic and therapeutic options available, as supported by the results of pivotal studies, different treatment regimens, and future management trends for both the dry and wet form of this important disease.
METHODS
This descriptive report offers a comprehensive analysis that focuses on the current “state of the art” literature, and highlights some plausible pathways for future treatments and approaches in the management of AMD.
RESULTS
The angiogenesis and inflammatory mediators, including vascular endothelial growth factors (VEGFs), play a major role in the development of CNV. VEGF-A, a 45 kDa homodimeric glycoprotein, belongs to the family that also includes VEGF-B through VEGF-E, platelet-derived growth factor (PDGF), and placental growth factor (PIGF). It is believed that VEGF-A is the most potent pro-angiogenic protein described to date, inducing proliferation and the sprouting and tube formation of endothelial cells. Intravitreal injection therapy with antiangiogenic agents is the most successful technique in the management of wet AMD and represents the first line of treatment2.
Currently, there are two drugs that have been approved by the US Food and Drug Administration (US-FDA) and BR-ANVISA, namely, ranibizumab (Lucentis; Genentech, US) and aflibercept (Eylea; Bayer, Germany). While awaiting FDA approval for ranibizumab, ophthalmologists began treating wet AMD with 1.25 mg bevacizumab, as this drug had a target specificity similar to that of ranibizumab11.
Bevacizumab (Avastin; Genentech, US) is a full-length recombinant monoclonal antibody that binds all VEGF isoforms. It was developed to inhibit pathological angiogenesis in tumors and is approved by the FDA for the treatment of metastatic colorectal cancer. In the field of ophthalmology, it is an off-label drug. There is a hypothesis that bevacizumab may be as proficient as ranibizumab in the treatment of wet AMD, and may offer a less expensive alternative to the approved substances that have been specifically adapted for intraocular administration. For a protocol of 12 fixed monthly injections, the projected cost of ranibizumab may be approximately $22.000 compared with approximately $600 for a bevacizumab-based therapy14.
Since its approval in July 2006, antiangiogenic therapy with 0.5 mg ranibizumab has been established as the basis for the management of wet AMD15,16. Ranibizumab is a recombinant, modified, Fab fragment of a monoclonal antibody. The Fc fragment was removed from the parent particle, which created an antibody that was one-third of the molecular weight of bevacizumab with a shorter half-life; consequently, ranibizumab has high safety parameters and good retinal penetration after intravitreal injection. Additionally, the affinity for VEGF of the compound was enhanced by modifications in a group of amino acids.
Aflibercept is a fusion protein that binds to VEGF-A and PIGF. It has high affinity and efficacy. The VIEW 1 and 2 clinical trials supported its approval. The Fc portion of lgG1 supports the “trap” configuration of this drug. This Fc part was intended not only to stabilize the protein’s structure, but also to create an acceptable plasma half-life. Administration of intravitreal aflibercept in monthly injections for the initial 3 months, followed by a bimonthly fixed regime, produced similar efficacy and safety outcomes as monthly ranibizumab. However, in the VIEW 1 study, monthly aflibercept provided statistically superior best-corrected visual acuity (BCVA). Several interventional studies have suggested that there may be a superior anatomic result with aflibercept compared with ranibizumab. The binding affinity to VEGF of intravitreal aflibercept is greater than the affinities of both bevacizumab and ranibizumab. This affinity may be associated with a higher efficacy or, as predicted by a mathematical model, with a substantially longer intraocular duration of action19.
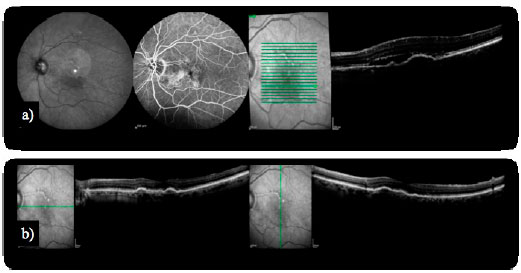
Some complications inherent to the clinical history of wet AMD have been presented as an adapted spectrum of therapies. A PED is the detachment of the sensory retina from the underlying Bruch’s membrane. Previous studies have shown that PEDs respond poorly to treatments for CNV, including photodynamic therapy (PDT) and antiangiogenic agents. With the introduction of aflibercept, PED involutions have been observed in patients with refractory AMD (Figure 2). Unfortunately, despite the reductions in PED dimensions and in the central retinal thickness (CRT) on OCT, this fact does not correlate with changes in vision20.
Since the pivotal results from the Minimally Classic/Occult Trial of the Anti-VEGF
Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) trials, treatment protocols have had to be adapted for daily clinical practice, and also had to accommodate the patient interests. Subjects who received fixed monthly ranibizumab injections during 24 months in these previous clinical trials continued for additional 24 months on a “pro re nata (PRN)” ranibizumab program in the Horizon protocol; the results demonstrated an overall decline of 8.2 letters over 7 to 8 year period. Macular atrophy was the most prominent chronic effect observed, and was present in virtually all subjects. This suggested some of the lifelong features of this disorder. At that time, it was unclear if fixed protocols with monthly intervals represented the best treatment approach. Clinicians then proposed that the need for retreatment was unpredictable and may vary among patients.
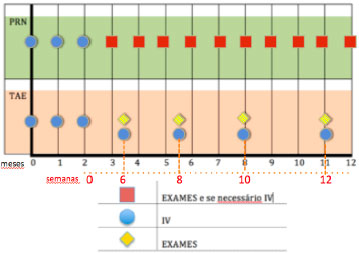
This consideration led to different treatment programs, such as the “PRN” (also called “as needed”), and the “treat-and-extend” (TAE) methods (Figure 3). It is important to highlight some relevant reports that employed a “PRN” protocol: CATT, PrONTO, and Harbor. Regillo defined the TAE strategy as a continuous dosing strategy with the aims of minimizing recurrences and maximizing long-term visual outcomes. At the same time, the dosing is variable and individualized; therefore, there is the potential to minimize overtreatment and maximize safety. The variable strategy is also cost-effective, because it minimizes the number of office visits, tests, and injections.
The CATT trial was a non-inferiority (NI) study that identified similar results for ranibizumab and bevacizumab in a monthly or in a “PRN” basis over a 24-month period. However, bevacizumab in a “PRN” regimen did not present the Nl point compared with a monthly ranibizumab regimen. The PRN treatment resulted in smaller gains in BCVA, whether the “PRN” regimen was instituted at enrollment or after 12 months of monthly treatment. In addition to the CATT study, other prospective trials, such as Inhibition of VEGF in Age-related choroidal Neovascularisation (IVAN) trial, compared both drugs under similar and different protocols. In IVAN, a European study similar to CATT, the 24-month results demonstrated that bevacizumab failed to fall within the Nl margin, as exhibited by the CATT trial, and that the reduction in the frequency of retreatment resulted in a small loss of efficacy, irrespective of the drug. In the PrONTO study, the PRN protocol established that retreatment was performed during the first 12 months if the OCT scan demonstrated an upsurge of at least 100 |jm in the CRT or a loss of 5 letters. By the end of 24 months, patients had improved by 11.1 letters and had received 9.9 injections over this period; this indicated that the patients enrolled in the PrONTO study had similar results to the MARINA and ANCHOR trials, but with fewer injections. The Harbor study group concluded that 2.0 mg ranibizumab did not perform better than the usual 0.5 mg, which was inconsistent with the Survival and Ventricular Enlargement (SAVE) trial results that had demonstrated better BCVA in patients who were treated with a higher dose. However, while the SAVE trial included patients with recalcitrant wet AMD, the Harbor trial only enrolled naïve treatment patients. Besides comparing fixed monthly ranibizumab treatments with a flexible “PRN” regime, Harbor was the first trial in which the investigators used a spectral-domain OCT (SD-OCT) technology, which provided a high-resolution optical ‘histology’. According to the turf trial results, patients with recalcitrant wet disease, who were already under treatment with 2.0 mg ranibizumab, maintained significant anatomic improvement when treated with 2.0 mg aflibercept.
Spaide and Freund were the first to describe a TAE protocol, and their group subsequently published two small retrospective reports that showed good visual outcomes. The TAE program allows for an individualized algorithm of treatment. Currently, this is the most commonly employed treatment approach in the United States, according to the American Society of Retina Specialists 2014 Membership Survey: Preferences and Trends, with approximately 77.8% of retina specialists favoring this form of dealing with wet AMD. This strategy comprises three initial injections on a monthly basis, followed by a particular program of subsequent injections, until deterioration is evident. The injection maintenance is considered an important attribute of the TAE algorithm so as to certify disease stability, and to minimize structural damage that may result from the recurrence of CNV activity (Figure 4).
DISCUSSION
Antiangiogenic therapy has been established as the mainstay for the treatment of wet AMD. Unfortunately, a significant part of patients are diagnosed, already with subretinal fibrosis, indicating that initial diagnosis is crucial to provide better results.
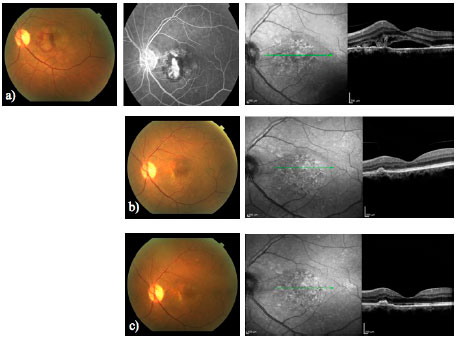
Imaging advances have made it possible to study outer retina abnormalities in a proper way. OCT technology has improved since it was described, and some of these advances may turn into helpful tools for clinical practice. The en-face OCT provides a coronal view of the posterior segment at different levels. The swept-source OCT employs a longer wavelength (1050 nm) than the time-domain OCT or the SD-OCT. This represents an advantage for studying the choroid. The higher scanning speed also captures images in a shorter time frame, enhancing the possibility of acquiring higher resolution photographs. Recent publications have demonstrated these applications and the correlation between angiography and en face SS-OCT in wet AMD; this may permit a better understanding of its physiopathology and help us with the management of our patients (Figure 5)28.
The concept of “at home” monitoring has been discussed as well. The PHP {3.1 [EN] Please expand the abbreviation} has exhibited high sensitivity in the detection of CNV. The Home study found that participants who utilized a home monitoring strategy not only had less vision loss at the moment of CNV detection than other patients, but also maintained a better BCVA.
A gap has developed between the labeling of drugs that are available for the treatment of wet AMD and their actual application in clinical practice. With the goal of improving our approach, there are a number of potential alternative strategies beyond the injection of available antiangiogenic agents with some still under investigation. Combination therapy of antiangiogenic therapy and ionizing radiation is referred as may be another option to reduce treatment frequency31. Avila et al found a gain in visual acuity of 8.9 letters with 91 % of patients maintaining their vision and 68% demonstrating stable or improved vision after 12 months. In addition, 70% of patients did not require an additional anti-VEGF injection. Combination therapies involving pharmacological agents have also been studied. An inhibitor of PDGF, called Fovista (E10030, Ophthotech) is being investigated in combination with an anti-VEGF agent. In a prior report, the combination therapy met the primary endpoint of superiority over an anti-VEGF monotherapy, and demonstrated a 62% additional benefit at all-time points. The search for better drugs and better delivery system is ongoing33.
Apart from the AREDS formulation, there is still no approved option for the treatment of dry AMD. The pathophysiology of this disorder shows that the aging process damages the retinal pigment epithelium (RPE), which loses the ability to manage photoreceptor metabolism. With time, the RPE, photoreceptors, and ultimately the choriocapillaris degenerate, leading to GA (34). Stem cell (SC) therapy represents a new therapeutic approach by which retinal degenerative diseases may be treated through the replacement or supplementation of the damaged RPE or photoreceptors. The human embryonic cells (hESC) and the induced pluripotent cells are the major forms of retinal SC therapy at this moment. Schwartz et al published the preliminary data on the first patient with GA treated with hESC-derived RPE in 2012.
Although significant advances have raised the possibility of using this therapy, substantial issues will need to be addressed before there are any successful upcoming trials34.
At present, two strategies to prevent dry AMD progression are being clinically investigated: visual cycle modulation to prevent photoreceptor and RPE loss and complement inhibition. The first modality is represented by the Emixustat HCI (ACU-4429, Acúcela). It is an orally-administered visual cycle modulator that has been designed to inhibit the activity of the visual cycle isomerase (RPE65), dropping the level of vitamin A processing during the visual cycle. RPE65 has been explored as a potential therapeutic target in preclinical models since the early 2000s. Lampalizumab, an anti-factor D (Genentech), is a selective inhibitor of the alternative complement pathway. This is an antigen-binding fragment of a humanized monoclonal antibody that inhibits complement factor D and blocks activation of the alternative complement pathway. It has demonstrated a reduction of 20.4% in GA 18 months after 10 mg lampalizumab injection36. Regillo et al presented the results of the MAHALO study in the 2013 Retina Subspecialty Day of the AAO Annual Meeting.
Squalamine (Ohr Pharmaceutical) is a topical option that inhibits VEGF, PDGF, and basic fibroblast growth factor (bFGF) and may decrease the number of anti-VEGF injections required by the patient. However, it has not been approved yet. A recent publication presented a study that compared the topical ocular formulation of pazopanib, a self-administered therapy for the treatment of wet AMD, with ranibizumab monotherapy. Pazopanib is a multi-target tyrosine kinase inhibitor that inhibits VEGF receptors1,2,3 as well as the PDGF pathway. A previous study showed that the topical ocular instillation of pazopanib “TID”{3.1 [EN] Please expand the abbreviation}was associated with improved BCVA in patients with subfoveal CNVs secondary to AMD. Pazopanib was well tolerated in this trial. Daily pazopanib drops in patients with wet AMD did not result in a therapeutic benefit beyond that benefits that was obtained with ranibizumab alone38.
In conclusion, despite the significant and constant advances in the treatment of wet AMD with antiangiogenic therapy, patients still require numerous injections and office visits. However, future therapies have the potential not only to reduce patient visits and injections, but also to improve outcomes by targeting additional pathways31. Nevertheless, AMD is a chronic disorder, and the consensus is that the best outcomes will be accomplished with prompt detection and treatment. The aim of the chosen therapy should be to diminish the growth of CNV and maintain the best vision possible for patients over a long period of time.
REFERENCES
1 Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Preditores de resposta ao tratamento anti-VEGF na degeneração macular neovascular relacionada à idade. Levantamento Oftalmológico. 2014;59(1):1 -18.
2 Padrão de Práticas Recomendadas pela Academia Americana de Oftalmologia (PPP - AAO) para a Degeneração Macular Relacionada à Idade. http://one.aao.ora/preferred-practice-pattern/aae-related-macular-deaeneration-ppp-2Q15. 2015.
3 Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Diretrizes da Sociedade Européia de Especialistas em Retina (EURETINA) para o manejo da degeneração macular neovascular relacionada à idade. Revista Britânica de Oftalmologia. 2014;98(9):1144-67.
4 Grupo de Estudos sobre Doenças Oculares Relacionadas à Idade (estudo 2). Luteína + zeaxantina e ácidos graxos omega-3 para a degeneração macular relacionada à idade: ensaio clínico randomizado do estudo 2 (AREDS2) de Doenças Oculares Relacionada à idade. Jama (Revista da Associação Médica Americana). 2013;309(19):2005-15.
5 Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Grupo S-USA. Resultados em pacientes após de tratamento com ranibizumab nos estudos ANCHOR, MARINA e HORIZON: um estudo de coorte multicêntrico (SEVEN-UP). Oftalmologia. 2013;120(11):2292-9.
6 Chew EY, Klein ML, Clemons TE, Agron E, Ratnapriya R, Edwards AO, et al. Ausência de associação clínica significativa entre os genotipos CFH e ARMS2 e resposta a suplementos nutricionais: relatório AREDS número 38. Oftalmologia. 2014;121 (11):2173-80.
7 Faes L, Bodmer NS, Bachmann LM, Thiel MA, Schmid MK. Acurácia diagnostica da Tela de Amsler e do perímetro de hiperacuidade preferencial na triagem de pacientes com degeneração macular relacionada à idade: revisão sistemática e meta-análise. Olho. 2014;28(7):788-96.
8 Khan S, Engelbert M,Imamura Y, Freund K. Vasculopatia polipoidal da coroide: Achados da Angiografia com Indocianina Verde de forma Simultânea e Rastreamento Ocular pela Tomografia de Coerência Óptica com Domínio Espectral. RETINA 2012; 32:1057-1068.
9 Keane PA, Patel PJ, Liakopoulos S, Heussen FM, Sadda SR, Tufail A. Avaliação da degeneração macular relacionada à idade pela tomografia de coerência ótica. Levantamento Oftalmológico. 2012;57(5):389-414.
10 de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhaes O, Jr, Penha FM, Farah ME. Citoquinas em degeneração macular neovascular relacionada à idade: fundamentos da terapia combinada alvo. Revista Britânica de Oftalmologia. 2011 ;95(12):1631 -7.
11 Grupo de Pesquisas CATT. Ranibizumab e Bevacizumab para Degeneração Macular Neovascular Relacionada à Idade. Rev Med N Engl. 2011 ¡364(20)1897-1908.
12 Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus Bevacizumab para a Degeneração Macular Neovascular Relacionada à Idade: Resultados do ensaio randomizado de não inferioridade GEFAL. Oftalmologia. 2013;120(11):2300-9.
13 Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. Ranibizumab versus bevacizumab para o tratamento da degeneração macular relacionada à idade: achados de um ano do ensaio randomizado IVAN. Oftalmologia. 2012;119(7):1399-411.
14 Jeng KW, Wilgucki J, Halperin S et al. ESPECIALISTAS EM RETINA RECOMENDAM PARA OS PACIENTES ABORDAGENS DIFERENTES DAS QUE ELES ESCOLHERIAM PARA SEU PRÓPRIO TRATAMENTO DE DEGENERAÇÃO MACULAR RELACIONADA À IDADE Retina 2014; 34:1796-1801.
15 Rosenfeld PJ, Heier JS et al. Ranibizumab para degeneração macular neovascular relacionada à idade. Rev Med N Engl. 2006(355):12.
16 Brown D KP, Michels M et al. Grupo de estudos ANCHOR. Ranibizumab versus Verteporfin para degeneração macular neovascular relacionada à idade. Rev Med N Engl. 2006(355): 1432-44.
17 Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Aflibercept intravítreo (Armadilha-Olho de VEGF) na forma úmida de degeneração macular relacionada à idade. Oftalmologia. 2012;119(12):2537-48.
18 Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Injeções intravitreas de flibercept para a degeneração macular neovascular relacionada à idade: resultados de noventa e seis semanas de estudos VIEW. Oftalmologia. 2014;121 (1):193-201.
19 Arcinue CA, Ma F, Barteselli G, Sharpsten L, Gomez ML, Freeman WR. Resultados de um ano de aflibercept para degeneração macular neovascular relacionada à idade recorrente ou persistente. Revista Americana de Oftalmologia. 2015;159(3):426-36 e2.
20 Broadhead G, Hong T, Zhu M et al. RESPOSTA DE DESPRENDIMENTOS DO EPITÉLIO PIGMENTÁRIO AO AFLIBERCEPT INTRAVÍTREO ENTRE PACIENTES COM DEGENERAÇÃO MACULAR NEOVASCULAR RELACIONADA À IDADE RESISTENTE A TRATAMENTO. Retina 2014; 0:1-7.
21 Grupo de Pesquisas Comparativas de Ensaios de Tratamento da Degeneração Macular Relacionada à idade, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab e bevacizumab para tratamento da degeneração macular neovascular relacionada à idade: resultados de dois anos. Oftalmologia. 2012;119(7):1388-98.
22 Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, et al. Progressão da Atrofia Macular e 7 anos de Resultados Visuais em Indivíduos dos Estudos ANCHOR, MARINA e HORIZON: o Estudo SEVEN-UP. Revista Americana de Oftalmologia. 2015.
23 Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. Regime de dose variável de ranibizumab intravítreo para a degeneração macular neovascular relacionada à idade: ano 2 do estudo PrONTO. Revista Americana de Oftalmologia. 2009;148(1):43-58 e1.
24 Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, et al. Eficácia de vinte e quarto meses e segurança do ranibizumab a 0,5 mg ou 2,0 mg em pacientes com degeneração macular neovascular relacionada à idade. Oftalmologia. 2014;121 (11 ):2181 -92.
25 Wykoff CC, Brown DM, Croft DE, Wong TP. Resultados de Dois Anos do SAVE: ranibizumab a 2,0 mg para DMRI neovascular recalcitrante. Oftalmologia. 2013;120(9):1945-6 e1.
26 Wykoff CC, Brown DM, Maldonado ME, Croft DE. Tratamento com aflibercept para a degeneração macular relacionada à idade exsudativa em pacientes com resposta incompleta a injeções múltiplas de ranibizumab (ensaio TURF). Revista Britânica de Oftalmologia. 2014;98(7):951 -5.
27 Rayess N, Houston SK, 3rd, Gupta OP, Ho AC, Regillo CD. Resultados após 3 anos de tratamento da degeneração macular neovascular relacionada à idade utilizando o regime ‘tratar e estender’. Revista Americana de Oftalmologia. 2015;159(1 ):3-8 e1.
28 Flores-Moreno I, Arias-Barquet L, Rubio-Caso MJ, Ruiz-Moreno JM, Duker JS, Caminal JM. Tomografia de coerência ótica com fonte de varredura ‘En face’ na degeneração macular neovascular relacionada à idade. Revista Britânica de Oftalmologia. 2015.
29 Isaac D, Ávila M, Cialdini A. Comparação da T de Amsler original com o perímetro de hiperacuidade preferencial para detecção de neovascularização da coroide na degeneração macular relacionada à idade. Arq Bras Oftalmol. 2007;70(5):771 -6.
30 Group AHSR: Chew EY, Clemons TE, Bressler SB, Elman MJ, Danis RP, et al. Ensaio randomizado de um sistema de monitoramento em casa para detecção precoce de neovascularização da coroide, estudo de monitoramento do olho em casa (HOME). Oftalmologia. 2014;121(2):535-44.
31 Kaiser P. Terapias emergentes para a degeneração macular neovascular relacionada à idade: drogas em fase de preparação. Oftalmologia. 2013;120(5 Supl):S11 -5.
32 Ávila MP, Farah ME, Santos A, Duprat JP, Woodward BW, Nau J. Segurança de curto prazo de doze meses e resultados de acuidade visual de um estudo prospectivo multicêntrico de braquiterapia epiretinal de estróncio-90 com bevacizumab para o tratamento de neovascularização subfoveal coroidal secundária à degeneração macular relacionada à idade. Revista Britânica de Oftalmologia. 2009;93(3):305-9.
33 Tolentino MJ, Dennrick A, John E, Tolentino MS. Drogas em ensaios clínicos de Fase II para o tratamento da degeneração macular relacionada à idade. Opinião de especialista em investigação de drogas. 2015;24(2):183-99.
34 Chakravarthy U, Soubrane G, Bandello F, Chong V, Creuzot-Garcher C, Dimitrakos SA, 2nd, et al. Evolução das diretrizes europeias para o controle da degeneração macular neovascular relacionada à idade. Revista Britânica de Oftalmologia. 2006;90(9):1188-96.
35 Kvanta A, Grudzinska MK. Tratamento baseado em células tronco para a atrofia geográfica: promessas e imprevistos. Ata Oftalmológica. 2014;92(1):21-6.
36 Garcia JM, Mendonça L, Brant R, Abud M, Regatieri C, Diniz B. Terapia de células tronco para doenças da retina. Rev Mundial Cel Tronco 2015; 7(1): 158-162.
37 Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Atrofia geográfica: características clínicas e abordagens terapêuticas potencias. Oftalmologia. 2014;121 (5):1079-91.
38 Csaky KG, Dugel PU, Pierce AJ, Fries MA, Kelly DS, Danis RP, et al. Avaliação Clínica do Pazopanib em Gotas Oculares versus Injeções Intravítreas de Ranibizumab em Indivíduos com Degeneração Macular Neovascular Relacionada à Idade. Oftalmologia. 2015;122(3):579-88.

Fonte de financiamento: declaram não haver.
Conflito de interesses: declaram não haver.
Received on:
April 23, 2015.
Accepted on:
May 18, 2015.