Lisandro Massanori Sakata
DOI: 10.17545/e-oftalmo.cbo/2016.53
RESUMO
OBJETIVO: Rever e atualizar os métodos disponíveis para a avaliação do ângulo da câmara anterior (ACA).
MÉTODOS: Analisamos e discutimos os achados mais significativos encontrados em uma seleção de artigos publicados sobre ACA e fizemos uma análise crítica de cada método. Resultados: A gonioscopia é o exame-padrão de referência mundial para ACA. Pelo fato de nenhuma outra técnica de exame permitir a diferenciação adequada entre fechamento de ângulo aposicional e fechamento de ângulo sinequial, a gonioscopia de indentação é essencial para decidir o manejo clínico mais adequado. Técnicas de imagem, como a biomicroscopia ultrassônica e a tomografia de coerência ótica do segmento anterior são modalidades úteis e promissoras para a avaliação de ACA; no entanto, os oftalmologistas necessitam de imagens de boa qualidade e compreensão das particularidades e limitações inerentes a estas técnicas para que possam interpretar corretamente os resultados.
CONCLUSÃO: As tecnologias de imagem podem contribuir significativamente para a avaliação clínica do ACA; no entanto, estudos longitudinais devem ser realizados para determinar os valores relativos dos achados no manejo de pacientes com fechamento do ângulo. Até que isso seja feito, nenhuma destas técnicas de imagem poderá substituir a gonioscopia como exame-padrão de referência.
Palavras-chave: Gonioscopia; Câmara anterior; Glaucoma de Ângulo Fechado.
ABSTRACT
OBJECTIVE: To review and update the available methods for evaluating the anterior chamber angle (ACA).
METHODS: We analyzed and discussed the main findings of selected published articles on ACA assessment and performed a critical review of each method. Results: Gonioscopy is the reference standard examination for evaluation of ACA. Because no other examination technique permits appropriate differentiation between apposition and synechial angle closure, indentation gonioscopy is essential to determine proper clinical management decisions. Imaging techniques such as ultrasound biomicroscopy and anterior segment-optical coherence tomography are useful and promising modalities for ACA assessment; however, ophthalmologists need to look for good-quality images and understand the particularities and inherent limitations of these techniques for proper interpretation of the results.
CONCLUSION: Imaging technologies may represent an important adjunct for the clinical assessment of ACA; however, longitudinal studies should be performed to determine the relative values of their findings in the management of patients with angle closure. Until then, none of these imaging techniques can replace gonioscopy as the reference standard examination.
Keywords: Gonioscopy; Anterior Chamber; Glaucoma, Angle-Closure.
INTRODUCTION
Primary angle-closure glaucoma (ACG) is a visually impairing type of glaucoma that accounts for approximately half of the blindness caused by this disease worldwide.1,2,3,4,5 The closure of the anterior chamber angle (ACA) seems to be the primary abnormality leading to intraocular pressure (IOP) elevation and glaucomatous optic neuropathy.6 The contact between the peripheral iris and the trabecular meshwork may impair aqueous humor drainage from the anterior chamber by pre-trabecular mechanisms due to the mechanical obstruction of the functional trabecular meshwork by the peripheral iris and/or by abnormalities at the ultrastructural level of the trabecular meshwork due to the prolonged friction/apposition of the iris against the angle wall.7
Thus, it is likely that iris-trabecular contact is an important risk factor in the development of this disease. Detection of appositional angle closure (temporary) is an essential step in the prevention of blindness due to ACG, as interventions such as iridotomy may halt the angle-closure process in most cases, preventing the development of glaucomatous optic neuropathy.
With this information, it is clear that a quick, reliable, and reproducible assessment of ACA is important. However, performing this evaluation may represent a major challenge in daily clinical practice. The aim of this paper was to review and update the available methods for ACA evaluation.
Current Reference Standard Examination: Gonioscopy
The current reference standard examination for evaluating ACA configuration is indirect gonioscopy, and it can be easily and quickly performed by adequately trained ophthalmologists. Gonioscopy is a non-expensive examination as it does not require sophisticated equipment. It enables a direct view of a broad part of the angle as well as identification of anatomical reference points (such as Schwalbe's line, pigmented posterior trabecular meshwork, and scleral spur), which permit a proper interpretation of the status of ACA opening.
More importantly, the dynamic part of this examination enables differentiation between appositional (temporary) and synechial (permanent) angle closure. This differentiation is essential to determine proper clinical management, and to date, no other technology is able to discern apposition from synechial angle closure other than indentation gonioscopy.
However, gonioscopy has many limitations. It requires considerable skill and experience, and the ACA assessment may be affected by many variables. Pressure of the gonio lens on the globe (inadvertent indentation) and light exposure to the pupils during the examination (miosis) may lead to an overestimation of the angle opening, resulting in underdetection of the eyes at the risk of angle closure. The ACA opening evaluation is subjective, and documentation of the findings may be difficult. Studies have shown that even experienced examiners demonstrated only moderate agreement in determining the angle width.8,9 Furthermore, gonioscopy is not performed or not properly performed by many ophthalmologists in routine clinical practice.
Because of the difficulties and limitations of the reference standard, other methods have been developed in an attempt to obtain more objective measures of ACA. Ultrasound biomicroscopy (UBM), the Scheimpflug imaging system, and anterior segment-optical coherence tomography (AS-OCT) are capable of high-resolution imaging of the anterior segment and have the potential to be used for evaluation of the cornea morphology,10,11 to plan the sizing of the phakic intraocular lenses, 12,13 and to assess the morphology of trabeculectomy blebs.14,15,16 Moreover, these devices are able to perform objective measures of ACA and provide a straightforward way to document the ACA configuration. This manuscript will provide a brief description of these techniques and review their roles in the evaluation of ACA.
Scheimpflug Imaging System
Scheimpflug systems such as Pentacam (Oculus, Lynwood, WA, USA) and Galilei (Ziemer, Wood River, IL, USA) enable anterior segment imaging using a camera perpendicular to the slit beam, which permits quick imaging of the anterior segment of the eye. This technique is very useful for ophthalmological care, particularly for cornea assessment. Further, as an adjunct function, Scheimpflug systems are able to obtain general anterior chamber measurements (central anterior chamber depth, anterior chamber area, and anterior chamber volume), which have a reasonable diagnostic value in identifying eyes at risk of angle closure. Of note, anterior chamber depth measurements can be obtained using simpler devices, and all eyes at risk of angle closure identified by this method must be evaluated by proper gonioscopy.
Nevertheless, Scheimpflug imaging systems are very limited for ACA evaluation. These systems use visible light during the imaging process (miosis), which may lead to overestimation of the angle width. However, the main drawback is that the characteristics of the images obtained at the region of the angle do not permit the identification of anatomical landmarks (i.e., scleral spur), and therefore, the interpretation of the angle opening status simply cannot be determined properly in many eyes with narrow or closed angles. Thus, Scheimpflug imaging systems are not suitable for proper ACA assessment.
Ultrasound Biomicroscopy
The UBM examination was presented by Pavlin et al. in 1991; and represents a non-invasive method for obtaining high-resolution images of the anterior segment of the eye. Generally, UBMs have a linear high-frequency ultrasonic transducer (50 MHz) that emits sound waves through ocular tissues, with a penetration depth of 5.5 mm, and detects their reflection from tissue interfaces.17 Thus, UBM is capable of obtaining in vivo cross-sectional images of the anterior segment structures of the eye with axial and transverse resolutions of approximately 25 µm and 50 µm, respectively.18,19
The UBM evaluation requires immersion techniques. The device probe must be immersed in a saline solution, which is usually kept in a small, transparent acrylic eyecup positioned between the eyelids. The examination is usually performed in the supine position, but recent versions of this technology allow the examination to be performed at the sitting position. Moreover, newer versions are able to acquire limbus-to-limbus images. Nevertheless, the UBM examination is a relatively time-consuming and unpleasant method for the patient compared with non-contact imaging methods.
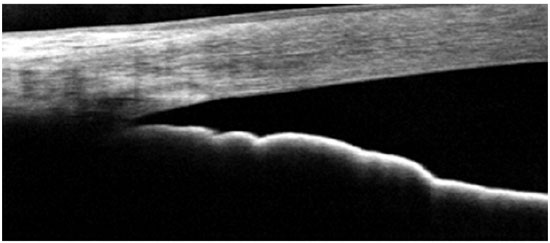
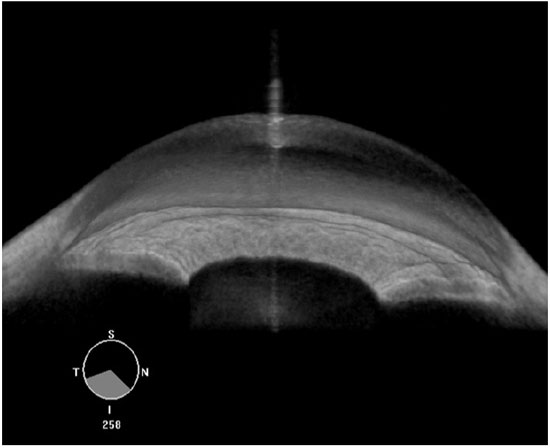
Compared with AS-OCT, UBM images quality may be considered more dependent on the examiner’s skills. For a proper ACA morphological evaluation, UBM images must be acquired perpendicularly to the structures of interest. A double arc on the cornea is one of the findings correlated with a properly acquired image (Figure 1). Regarding angle assessment, the frozen radial section of the drainage angle should aim to include a well-defined scleral spur, which is important for the proper interpretation of the ACA opening.
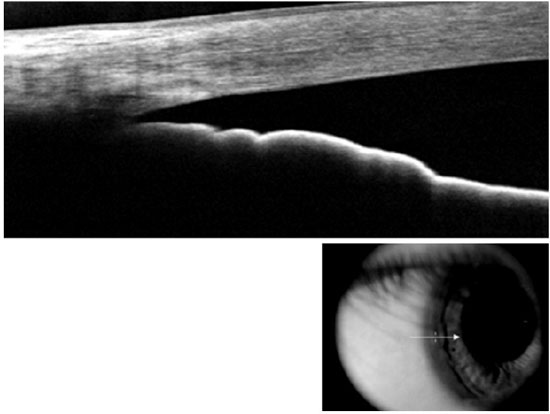
Nevertheless, when properly acquired, UBM images can provide high-quality information about the ACA opening. Furthermore, UBM is currently the only technique that is capable of imaging the structures behind the iris (i.e., the ciliary processes and iris cysts), whereas the light waves used in AS-OCT are blocked by the sclera and pigment epithelium of the iris.
Anterior Segment-Optical Coherence Tomography
OCT was first reported in 1991,20 and it was first used in ophthalmology for imaging of the posterior segment of the eye. Analogous to ultrasound, this technology obtains in vivo cross-sectional images of tissues, but uses light waves to obtain the reflectivity profile of the structure under investigation. AS-OCT is a rapid, non-contact method for obtaining real-time images of ACA.
Posterior segment-OCT uses a 830-nm superluminescent diode as the light-emitting source, and no contact medium is required for obtaining the images.21,22 The wavelength of light used in AS-OCT was changed to 1310 nm as it allows increased penetration through scattering ocular structures, such as the sclera and iris, resulting in a more detailed visualization of the ACA morphology.22,24,25
Notably, light used by AS-OCT systems cannot image the anterior segment through the eyelids. Thus, the superior and inferior eyelids must be gently moved out of the way before obtaining AS-OCT scans of the superior and inferior ACA, respectively.
After acquisition, the scanned images of both devices have to be processed using a custom software. This image-processing software (“dewarping” software) compensates for the index of refraction transition at the air-tear interface and for the different group indices in the air, cornea, and aqueous humor to correct the physical dimensions of the images.12
Different OCT techniques for anterior segment imaging are currently available:
- Time-domain (TD)-OCT: Visante OCT (Carl-Zeiss Meditec, Dublin, CA, USA); slit-lamp (SL)-OCT (Heidelberg Engineering, Heidelberg, Germany)
- Fourier-domain (FD)-OCT: Cirrus (Carl-Zeiss Meditec); Spectralis (Heidelberg Engineering); RTvue OCT (Oculus Technology, Wynwood, WA, USA)
- Swept-source (SS)-OCT: Casia SS-1000 OCT (Tomey, Nagoya, Japan); Topcon DRI (Topcon Corp, Tokyo, Japan)
The TD AS-OCT technique obtains 2000 A-scans per second, with a maximum resolution of 60-75 µm and an axial resolution of 1025 µn, and it is able to provide limbus-to-limbus images of the anterior chamber. One drawback is that these devices were developed to provide anterior segment imaging only, and not posterior segment OCT imaging.
The FD-OCT devices are widely available in Brazil, and it is capable imaging of both the posterior and anterior segments of the eye. The scan speed varies depending on the device type: 26,000 A-scans per second for Optovue; 27,000 A-scans per second for Cirrus; and 40,000 A-scans per second for Spectralis. These devices are able to provide normal-resolution limbus-to-limbus imaging of ACA as well as high-resolution imaging of a single quadrant of ACA (Figures 2-4). However, to the best of our knowledge, to date, none of these three devices have incorporated the dewarping software for high-resolution OCT imaging of ACA. Thus, patients have to shift their eye position in order to keep the OCT beam perpendicular to the area of interest, so that ACA is centered on the image printout.
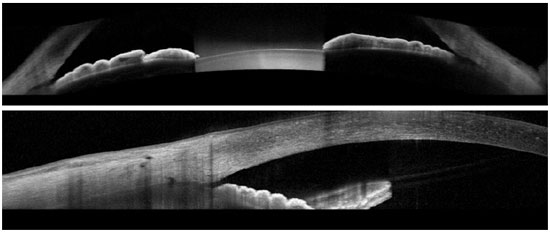
The most recent AS-OCT available is SS-OCT; whereas Casia was designed for anterior segment imaging only, the Topcon DRI is able to image both the anterior and posterior segments. The image resolution of the Casia OCT can reach 10 micra (axial) and 30 micra (transversal), with a scanning speed of 100,000-450,000 A-scans per second. It can scan the entire 360° of ACA in just a few seconds and is able to provide limbus-to-limbus images, high-resolution ACA images, and three-dimensional (3D) reconstruction of ACA (Figure 5).
Interpretation of Images and Anatomical Reference Points
ACA on AS-OCT images can be evaluated qualitatively by subjectively determining the open or closed status of ACA, and can be evaluated quantitatively by objectively measuring the ACA parameters.
Similar to UBM imaging analysis, interpretation of the ACA configuration in AS-OCT imaging (qualitative and quantitative) depends on determining the scleral spur location. The scleral spur is usually located on the widest part of the sclera,19 appearing as an inward scleral protrusion where some of the fibers of the longitudinal bundle of the ciliary muscle are attached. It is an anatomical landmark that reveals the relative location of the trabecular meshwork, which is located approximately 250-500 µm above the scleral spur along the angle wall.19
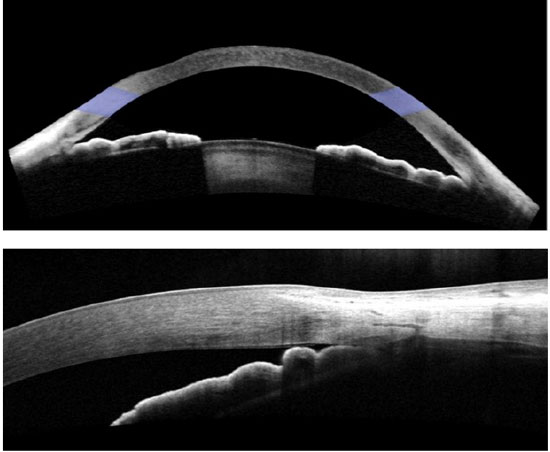
Quantitative analysis is performed using specific software such as UBM Pro2000 or Zhongshan Angle Assessment Program software for Visante OCT.26 During semi-automated quantitative analysis, the examiner needs to point out the exact location of the scleral spur, and the software automatically provides several ACA measurements (Figure 6). Commonly used parameters include the angle opening distance (AOD), which is the distance between the angle wall and iris along a line perpendicular to the trabecular meshwork and cornea, and the angle recess area (ARA), which is area of space between the line taken from AOD and the angle recess.19

AS-OCT and some new UBM devices are able to image the entire cross section of the anterior segment in one image frame, which represents an interesting method for assessing and documenting the profile of the iris and its relationship to the other anatomical parameters of the eye. Another interesting parameter is the lens vault or lens rise, which is a measure of how anteriorly the anterior surface of the lens is located relative to a line joining scleral spur to scleral spur. Studies have reported that both the lens vault and iris curvature were independently associated with angle closure, as defined on gonioscopy examination. However, it is important to note that neither of these parameters alone should determine the clinical management decisions, as angle closure results from a combination of several anatomical features in each eye.
While the interpretation of ACA images obtained using UBM also depends on the identification of the scleral spur, no study has directly addressed this issue. In a previous study that compared UBM with a prototypical version of TD AS-OCT, the authors stated that scleral spurs were more distinct in AS-OCT images, although no further information was provided.25 However, the ability to identify the scleral spur in UBM images is highly dependent on the examiner’s expertise, and when properly acquired, this structure may be better identified in a greater number of subjects in UBM images if compared to TD AS-OCT images.
Sakata et al.27 observed that the scleral spur location could not be identified in almost 30% of the AS-OCT images obtained using Visante OCT (TD). This difficulty was worse in eyes with narrow angles and in the superior and inferior quadrant images. Notably, almost 90% of the images could still be graded qualitatively by glaucoma specialists as having either an open or closed ACAs. Nevertheless, this problem of determining the scleral spur location may hamper quantitative analysis of ACA parameters in a substantial proportion of subjects. 27
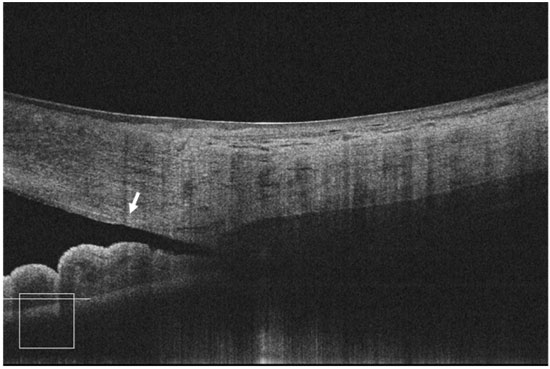
As technology evolves, high-resolution images obtained using FD-OCT have greatly improved image resolution and enable the identification of a new anatomical landmark—Schwalbe’s line (Figure 7). This structure can be observed in more than 90% of the images.41 This represents a relevant improvement as we can now identify the anterior and posterior boundaries of the trabecular meshwork in many eyes. The interpretation of qualitative and quantitative analyses is possible in most eyes because we can use one or both anatomical landmarks—the scleral spur and/or Schwalbe’s line.
As previously mentioned, all AS-OCT scanned images have to be corrected for the deviation of the scanning light crossing air, cornea, and aqueous humor because each medium has a different refractive index (“dewarping” process).
As previously mentioned, all AS-OCT scanned images have to be corrected for the deviation of the scanning light crossing air, cornea, and aqueous humor because each medium has a different refractive index (“dewarping” process).12 Notably, limbus-to-limbus AS images are corrected for these distortions; however, high-resolution images obtained using all FD-OCT devices are not. That is the reason why patients have to move their gaze in order to obtain angle images, so the light will scan the angle mostly in perpendicular way, minimizing distortions (Figure 2). As a simple quality-control parameter, the angle region must be centered on the printout of the high-resolution FD-OCT images. Images obtained using the Casia SS-OCT are dewarped, but to the best of our knowledge to date, only for the distortion arising from the air-cornea interface and not for the cornea-aqueous humor interface.
Another important point is that because both UBM and AS-OCT acquire cross-sectional images of ACA, some peculiarities in interpreting their results should be noted. As these images represent frozen radial sections of the drainage angle, they only reflect the ACA morphology of the scanned region of the angle, and, in some cases, this region may not be representative of the ACA morphology of the remaining portions of ACA. From this perspective, gonioscopy is superior to these technologies as the examiner has a broader view of the angle (whole quadrant).
The morphology and position of the ciliary processes observed on UBM images may vary in different radial scans (through a typical ciliary process or the valley between the ciliary processes).28 Slight differences in the acquisition location of the radial scans may influence the interpretation of the results, and perhaps, it should be recommended that examiners obtain radial sections passing through a typical ciliary process (probably better than obtaining scans randomly). Sakata and Malta29 showed that the image acquisition technique may affect the diagnosis of plateau iris mechanism. Using the same rationale, eyes with isolated peripheral anterior synechiae may have a similar confounding effect for both UBM and AS-OCT. A radial scan through a peripheral anterior synechia would show a closed angle, whereas that obtained immediately next to it (with no peripheral anterior synechia) could show a wider angle. Thus, for proper interpretation of the ACA configuration, these inherent limitations should be taken into consideration, and imaging results must be correlated with the gonioscopy findings.
The new SS AS-OCT enables a quite faster image acquisition when compared to previous technologies, minimizing motion artifacts. The Casia AS-OCT can scan the entire 360° of ACA in under 3 seconds. This device’s software is even able to build a 3D reconstruction of the anterior chamber. Other softwares are being developed to evaluate angle opening throughout 360°, and provide an overall view of the extent of the closed angle. This quick and more comprehensive information of the ACA provided by this new technology is promising, and future studies should assess its utility in routine clinical practice.
Literature Review
Reproducibility
Previous studies have evaluated the reproducibility of ACA measurements in the UBM and TD AS-OCT images. Measurement variability can be influenced by variability in image acquisition and also by image analysis due to the variability in subjectively determining anatomical landmarks, such as the location of the scleral spur. Thus, measurement variability represents an important step in the validation process of a new technology.
There are only a few studies that have evaluated the reproducibility in measuring ACA parameters using UBM.30,31 Both studies reported good reproducibility of anterior segment measurements obtained using UBM images; however, both evaluated eyes with open ACA only. The reproducibility of angle measurements in eyes with narrow angle was not assessed and this may not be as good as in open angles. As the location the location of the scleral spur is less detectable in narrow-angle eyes,27,32 the variability of these angle measurements is also likely to be higher in such eyes.
Regarding the reproducibility of ACA parameter measurements using TD AS-OCT, some papers have evaluated prototype versions of these devices.33,34 More recently, Leung et al.35 evaluated the agreement and inter-observer reproducibility of angle measurements obtained using Visante and SL-OCT. Images of the nasal and temporal angles were obtained in 49 eyes of healthy, normal subjects. The pupil diameter, AOD, trabecular-iris space area, and trabecular-iris angle parameters were measured by two experienced observers using semi-automated analysis software, unique for each instrument. These comprised a computer program developed by the author for Visante OCT images and the SL-OCT built-in analysis software. The study observed that both AS-OCT devices demonstrated good interobserver reproducibility for angle measurements. However, most participants included in their study had open angles, as assessed using gonioscopy, with a mean Shaffer grading of 3.71 ± 0.46 for the nasal angle and 3.46 ± 0.54 for the temporal angle. As mentioned previously, the reproducibility of angle measurements in narrow-angle eyes may not be as good as in open-angle eyes. Interestingly, when the authors compared the anterior segment parameters measured using Visante OCT and SL-OCT, they found poor agreement between the two devices. Differences in image acquisition (state of accommodation, exact scan location, and scanning speed) and image processing (algorithms for image dewarping and resolution of the scans) between Visante OCT and SL-OCT may help to explain this finding. Therefore, clinicians should be aware of the potential differences in angle measurements obtained with these devices.35
Console et al.26 evaluated the reproducibility of the commercially available Visante OCT in eyes with open and narrow ACAs. The authors observed that the reproducibility was worse in eyes with narrow ACA, and the determination of the scleral spur location by different examiners was an important source of variability.26
Comparison with gonioscopy
Barkana et al.36 compared the agreement between gonioscopy and UBM imaging in detecting appositional angle closure in 17 eyes with narrow ACA. The authors concluded that there was a high level of agreement between the two methods; furthermore, they found that performing gonioscopy in a completely dark room is important to avoid misdiagnosis of eyes at risk of angle closure. Notably, the study sample was rather small and included highly selected patients with narrow ACA only. It is possible that the good level of agreement between the methods may not be observed in a less biased population, such as in community-based or population-based studies. Indeed, even hospital-based studies, such as the one performed by Spaeth et al. in 22 patients with varied angle width, showed some discrepancies in appositional angle closure detected using gonioscopy and UBM.37
Nolan et al.38 compared the performance of the prototype version of the Visante OCT with that of gonioscopy in detecting angle closure. The authors observed that time-domain AS-OCT imaging was detecting more closed ACA than gonioscopy. In this study, an ACA was classified as closed on gonioscopy if the posterior trabecular meshwork could not be seen. A closed ACA on AS-OCT imaging was defined as the presence of any contact anterior to the scleral spur between the iris and angle wall. Thus, Nolan et al. 38 hypothesized that the disagreement between these two techniques is partially explained by the fact that while AS-OCT uses infrared light and does not require contact with the eye, inadvertent indentation and excessive light during gonioscopy may artificially open ACA. The authors stated that despite efforts to use as little light as possible and to minimize the length of the slit beam during gonioscopy, the anterior segment and pupil are still exposed to some light during the examination. This small amount of light may be sufficient to open up an angle that would be closed in the dark. In addition, Nolan et al. 38 also hypothesized that the anatomical landmarks considered in the two techniques are not the same. The authors stated that whereas it is possible to visualize landmarks such as Schwalbe's line and the posterior area of the trabecular meshwork using gonioscopy, the anterior boundaries of the trabecular meshwork are more difficult to identify using AS-OCT. Because the position of the scleral spur is easier to determine and the trabecular meshwork lies anterior to this structure, angle closure on AS-OCT was defined as the presence of any contact between the iris and angle structures anterior to the scleral spur. In gonioscopy, angle closure requires apposition between the iris and entire extent of the posterior trabecular meshwork. Thus, even if there is only a small area of contact between the iris and angle wall, these cases are defined as having closed angles on imaging.32,38,39
Sakata et al. 39 confirmed Nolan et al.'s 38 findings using the commercially available Visante OCT in a different study sample with different gonioscopy and AS-OCT image examiners. The authors also observed that higher number of cases of closed ACA was detected using AS-OCT than using gonioscopy mainly in the superior and inferior quadrants.
Further, even high-resolution images obtained using FD-OCT showed the same trend of higher number cases of closed angle detected on OCT than on gonioscopy, as observed in studies with relatively small sample size.41
Thus, it seems that the particularities in the methods of assessing and interpreting the ACA configuration of each technique may account for some of the discrepancies between gonioscopy and AS-OCT. It is unclear whether AS-OCT is overdetecting angle closure, or it is actually detecting eyes missed by gonioscopy. Notably, the rates of angle closure detected using AS-OCT are far greater than the actual prevalence of primary angle closure or primary ACG, even in Asians. Nevertheless, before becoming widely accepted in clinical practice, new technologies need to be validated against the existing standard. However, the potential limitations of the reference standard technique may affect the evaluation of the new techniques. Longitudinal studies are required not only to determine whether eyes classified as closed using AS-OCT only are indeed at the risk of developing ACG but also whether those classified as closed using AS-OCT will actually develop the disease.
Comparison with UBM
Radhakrishnan et al.25 compared the agreement in identifying narrow ACA using quantitative imaging by UBM and a prototype version of Visante OCT. This study evaluated a small sample of 17 normal subjects and 7 subjects with a narrow ACA as determined by gonioscopy. The assessment of the ACA in images obtained in the nasal and temporal quadrants was performed quantitatively by measuring several ACA parameters (angle opening distance, angle recess area, trabecular-iris space area, trabecular-iris contact length). The authors observed that both methods had similar discriminatory power to detect eyes with narrow ACA. Both devices provided similar mean values for the various ACA parameters, and when a significant difference was present (ARA at 500 µm and 750 µm; trabecular-iris space area at 750 µm), UBM tended to give smaller measurements.
Dada et al.40 evaluated 63 subjects with normal eyes and observed a good correlation between TD AS-OCT and UBM measurements obtained in the nasal and temporal quadrants. However, the authors did not provide the gonioscopy data for the study sample, and the comparison between the devices in eyes with narrow ACA remains to be evaluated.
Therefore, only a few studies have compared UBM and AS-OCT, with the limitations of small sample size and/or highly selective inclusion/exclusion criteria and/or evaluation of eyes with open ACA only. Further studies comparing these methods are needed to assess whether the results are interchangeable.
CONCLUSIONS
In summary, ACA evaluation is essential for the proper management of patients at risk of angle closure and for the prevention of blindness caused by ACG. Unfortunately, this assessment is not performed or is poorly performed by many clinicians. Gonioscopy is the reference standard examination for evaluating the angle opening. Moreover, its ability to differentiate appositional from synechial closure is essential to determine the proper clinical management. No other technology is able to adequately provide this information. Thus, improve gonioscopy training worldwide is the best and right option, however, this process will take a considerable deal of time and efforts.
Nevertheless, a report from the American Academy of Ophthalmology in 2013 concluded that although these imaging technologies provide useful information in the evaluation of the angle, none of them provide sufficient information about the ACA anatomy to be considered a substitute for gonioscopy.42 Longitudinal prospective studies are required to determine the relative value of AS-OCT findings in the management of patients with angle closure. In the future, these devices may prove to be useful for detecting eyes at risk for angle closure, and perhaps, they may permit discriminatory analysis to identify not only eyes at risk, but who indeed have a high chance for developing angle closure disease and ACG. Until then, it is important to emphasize that gonioscopy is a required feature of an eye examination, and glaucoma cannot be evaluated or patient treated properly without it being performed.
REFERENCES
1 Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol 1996;114:1235-41. https://doi.org/10.1001/archopht.1996.01100140435011
2. Foster PJ, Oen FT, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 2000;118:1105-11. https://doi.org/10.1001/archopht.118.8.1105
3. Dandona L, Dandona R, Mandal P, et al. Angle-closure glaucoma in an urban population in southern India. The Andhra Pradesh eye disease study. Ophthalmology 2000;107:1710-6. https://doi.org/10.1016/S0161-6420(00)00274-8
4. Foster PJ, Johnson GJ. Glaucoma in China: How big is the problem? Br J Ophthalmol 2001;85:1277-1282. https://doi.org/10.1136/bjo.85.11.1277
5. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262-7. https://doi.org/10.1136/bjo.2005.081224
6. Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol 2002;17:50-8. https://doi.org/10.1076/soph.17.2.50.14718
7. Sihota R, Lakshmaiah NC, Walia KB, Sharma S, Pailoor J, Agarwal HC. The trabecular meshwork in acute and chronic angle closure glaucoma. Indian J Ophthalmol 2001;49:255-9. PMid:12930118
8. Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, Baasanhu J. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol 2000;84:1255-9. https://doi.org/10.1136/bjo.84.11.1255
9. Foster PJ, Devereux JG, Alsbirk PH, et al. Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol 2000;84:186-92. https://doi.org/10.1136/bjo.84.2.186
10. Fine IH, Hoffman RS, Packer M. Profile of clear corneal cataract incisions demonstrated by ocular coherence tomography. J Cataract Refract Surg 2007;33:94-7. https://doi.org/10.1016/j.jcrs.2006.09.016
11. Lai MM, Tang M, Andrade EM, et al. Optical coherence tomography to assess intrastromal corneal ring segment depth in keratoconic eyes. J Cataract Refract Surg 2006;32:1860-5. https://doi.org/10.1016/j.jcrs.2006.05.030
12. Goldsmith JA, Li Y, Chalita MR, et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology 2005;112:238-44. https://doi.org/10.1016/j.ophtha.2004.09.019
13. Baikoff G. Anterior segment OCT and phakic intraocular lenses: a perspective. J Cataract Refract Surg 2006;32:1827-35. https://doi.org/10.1016/j.jcrs.2006.08.025
14. Singh M, Chew PT, Friedman DS, et al. Imaging of Trabeculectomy Blebs Using Anterior Segment Optical Coherence Tomography. Ophthalmology 2006.
15. Leung CK, Yick DW, Kwong YY, et al. Analysis of bleb morphology after trabeculectomy with the Visante anterior segment optical coherence tomography. Br J Ophthalmol 2006.
16. Muller M, Hoerauf H, Geerling G, et al. Filtering bleb evaluation with slit-lamp-adapted 1310-nm optical coherence tomography. Curr Eye Res 2006;31:909-15. https://doi.org/10.1080/02713680600910528
17. Nolan W. Anterior segment imaging: ultrasound biomicroscopy and anterior segment optical coherence tomography. Curr Opin Ophthalmol 2008;19:115-21. https://doi.org/10.1097/ICU.0b013e3282f40bba
18. Pavlin CJ. Practical application of ultrasound biomicroscopy. Can J Ophthalmol 1995;30:225-9. PMid:7585316
19. Pavlin CJ, Foster FS. Ultrasound biomicroscopy of the eye. New York, 1995:1-214. https://doi.org/10.1007/978-1-4612-2470-9
20. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254:1178-81. https://doi.org/10.1126/science.1957169
21. van Velthoven ME, Faber DJ, Verbraak FD, van Leeuwen TG, de Smet MD. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res 2007;26:57-77. https://doi.org/10.1016/j. preteyeres.2006.10.002
22. Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol 1994;112:1584-9. https://doi.org/10.1001/archopht.1994.01090240090031
23. Hoerauf H, Gordes RS, Scholz C, et al. First experimental and clinical results with transscleral optical coherence tomography. Ophthalmic Surg Lasers 2000;31:218-22. PMid:10847499
24. Radhakrishnan S, Rollins AM, Roth JE, et al. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 2001;119:1179-85. https://doi.org/10.1001/archopht.119.8.1179
25. Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 2005;123:1053-9. https://doi.org/10.1001/archopht.123.8.1053
26. Console JW, Sakata LM, Aung T, Friedman DS, He M. Quantitative analysis of anterior segment optical coherence tomography images: the Zhongshan Angle Assessment Program. Br J Ophthalmol 2008;92:1612-6. https://doi.org/10.1136/bjo.2007.129932
27. Sakata LM, Lavanya R, Friedman DS, et al. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol 2008;126:181-5. https://doi.org/10.1001/archophthalmol.2007.46
28. Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol 1992;113:390-5. https://doi.org/10.1016/S0002-9394(14)76160-4
29. Sakata LM, Sakata K, Susanna R, Jr., et al. Long ciliary processes with no ciliary sulcus and appositional angle closure assessed by ultrasound biomicroscopy. J Glaucoma 2006;15:371-9. https://doi.org/10.1097/01.ijg.0000212251.72207.19
30. Tello C, Liebmann J, Potash SD, Cohen H, Ritch R. Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest Ophthalmol Vis Sci 1994;35:3549-52. PMid:8056531
31. Souza Filho EC, Marigo Fde A, Oliveira C, Cronemberger S, Calixto N. [Intraobserver reproducibility in anterior segment morphometry of normal eyes using ultrasound biomicroscopy (UBM)]. Arq Bras Oftalmol 2005;68:177-83. https://doi.org/10.1590/S0004-27492005000200005
32. Nolan W. Anterior segment imaging: identifying the landmarks. Br J Ophthalmol 2008;92:1575-6. https://doi.org/10.1136/bjo.2008.146175
33. Muller M, Dahmen G, Porksen E, et al. Anterior chamber angle measurement with optical coherence tomography: intraobserver and interobserver variability. J Cataract Refract Surg 2006;32:1803-8. https://doi.org/10.1016/j.jcrs.2006.07.014
34. Radhakrishnan S, See J, Smith SD, et al. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci 2007;48:3683-8. https://doi.org/10.1167/iovs.06-1120
35. Leung CK, Li H, Weinreb RN, et al. Anterior chamber angle measurement with anterior segment optical coherence tomography: a comparison between slit lamp OCT and Visante OCT. Invest Ophthalmol Vis Sci 2008;49:3469-74. https://doi.org/10.1167/iovs.07-1477
36. Barkana Y, Dorairaj SK, Gerber Y, Liebmann JM, Ritch R. Agreement between gonioscopy and ultrasound biomicroscopy in detecting iridotrabecular apposition. Arch Ophthalmol 2007;125:1331-5. https://doi.org/10.1001/archopht.125.10.1331
37. Spaeth GL, Aruajo S, Azuara A. Comparison of the configuration of the human anterior chamber angle, as determined by the Spaeth gonioscopic grading system and ultrasound biomicroscopy. Trans Am Ophthalmol Soc 1995;93:337-47; discussion 347-51. PMCid:PMC1312064
38. Nolan WP, See JL, Chew PT, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology 2007;114:33-9. https://doi.org/10.1016/j.ophtha.2006.05.073
39. Sakata LM, Lavanya R, Friedman DS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology 2008;115:769-74. https://doi.org/10.1016/j.ophtha.2007.06.030
40. Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg 2007;33:837-40. https://doi.org/10.1016/j.jcrs.2007.01.021
41. Wong HT, Lim MC, Sakata LM, et al. High-definition optical coherence tomography imaging of the iridocorneal angle of the eye. Arch Ophthalmol 2009;127:256-60. https://doi.org/10.1001/archophthalmol.2009.22
42. Smith SD, Singh K, Lin SC, Chen PP, Chen TC, Francis BA, Jampel HD. Evaluation of the anterior chamber angle in glaucoma: a report by the american academy of ophthalmology. Ophthalmology. 2013 Oct;120(10):1985-97. https://doi.org/10.1016/j.ophtha.2013.05.034

Fonte de financiamento: declaro não haver.
Conflito de interesses: declaro não haver.
Recebido em:
11 de Maio de 2016.
Aceito em:
11 de Maio de 2016.