Carlos A. Moreira Jr1,2; Carlos A. Moreira Neto2,3; Jacó Lavinski4
DOI: 10.17545/eOftalmo/2023.0008
ABSTRACT
The authors have provided a review of central serous choroidopathy (CSC), showing details of its pathophysiology, clinical presentation, and imaging exams that well define the disease. They have also commented on the various types of treatment, emphasizing photodynamic therapy and other types of laser, specifically the red wavelength laser.
Keywords: Coroidoretinopathy; Stress; Corticoid, Laser; Photodynamic therapy.
RESUMO
Os autores fazem uma revisão sobre coroidopatia central serosa, mostrando detalhes de sua fisiopatologia, apresentação clínica e exames de imagem que bem definem a doença. Comentam ainda sobre os diversos tipos de tratamento, dando ênfase à terapia fotodinâmica e outros tipos de laser, especificamente o laser de comprimento de onda vermelha.
Palavras-chave: Coroidoretinopatia; Estresse; Corticoide; Laser; Terapia fotodinamica.
INTRODUCTION
Modern life has brought new challenges, often with more anxiety and emotional stress. Therefore, diseases that have an emotional background, such as central serous chorioretinopathy (CSC), have become more frequent. Thus, it is important that ophthalmologists and retinologists have good knowledge about CSC.
This pathology, formerly known as central serous retinopathy, is now known as central serous chorioretinopathy (CSC) due to the proven involvement of the choroid and retina.
The disease is characterized by the presence of one or more points of contrast leakage at the level of the retinal pigmented epithelium (RPE), leading to fluid accumulation in the posterior pole and resulting in serous retinal detachment.
There are two forms of the disease: acute, in which there is spontaneous resolution within a few weeks, and chronic, in which recurrent and/or persistent episodes of leakage lead to RPE atrophy and permanent visual impairment.
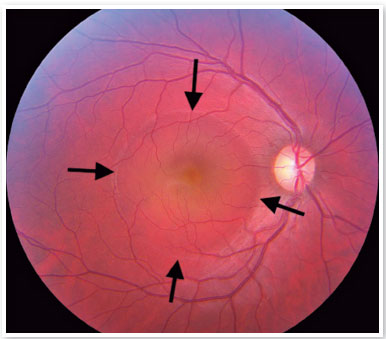
Although the pathophysiology is not well understood, there may be dysregulation of the choriocapillary microcirculation, leading to fluid leakage into the space just below the RPE, damaging and reducing the activity of these cells. The fluid accumulation can eventually cross the RPE and reach the subretinal space (Figure 1). Usually, spontaneous reabsorption of the fluid occurs. However, if microvascular dysregulation remains, the process will be perpetuated, resulting in recurrences1.
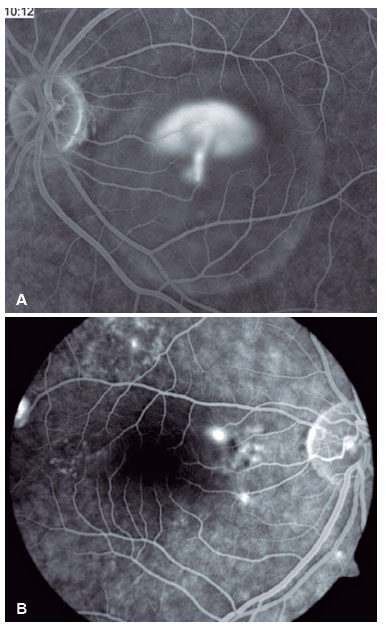
Fluorescein angiography (FA) is able to highlight these extravasation foci (single or multiple) that lead to detachment of the RPE and/or the neurosensory retina, characterized by CSC (Figure 2).
Indocyanine green is able to demonstrate multiple areas of increased vascular permeability in the choroid. This increased permeability could elevate tissue hydrostatic pressure, leading to major damage to the RPE and consequently allowing fluid to pass into the subretinal space2.
Increased serum levels of corticosteroids and catecholamines are also related to CSC3. Stress episodes, type A personalities, Cushing’s disease, the gestational period, and even chronic use of corticosteroids are involved in the pathogenesis, although the mechanism is not yet well defined. It is believed that they may influence the regulation of blood flow in the choroid by vasoconstriction, influencing the regulation of nitric oxide, and increasing capillary permeability or fragility4.
Initially, it was believed that there was a much higher incidence of cases in men between the ages of 30 and 50. However, in 1996, Spaide et al. concluded in a study of 130 patients that the ratio was only 2.6:1, and more than half were older than 50 years of age. Whites, Hispanics, and Asians are among the most affected races, with blacks being less affected6.
SYMPTOMS AND CLINICAL FINDINGS
Most symptoms include visual changes ranging from 20/20–20/200, and some patients may only have changes on the Amsler chart. Among the most frequent manifestations, we find complaints such as metamorphopsia, micropsia, dyschromatopsia, central scotoma, reduced contrast sensitivity, or even hypermetropization, mainly due to anterior retinal displacement.
On fundus biomicroscopy examination, we found neurosensory retinal elevations in the posterior pole of varying sizes. In addition, there is a loss of the foveal reflex, which eventually takes on a yellowish coloration due to the presence of retinal xanthophyll, which becomes more visible.
Adjacent to the detached neurosensory retina, the RPE is also detached, usually in a superior position. With the advent of optical coherence tomography (OCT), it has been possible to identify a higher incidence of RPE detachments and characterize their shapes. In addition, through histopathological studies, the presence of subretinal and sub-RPE fibrin and subretinal lipids have also been identified, especially in recurrent or chronic cases7.
Some cases may present with a peripheral detachment of the neurosensory retina, in which intense or prolonged extravasation in the posterior pole can lead to inferior fluid accumulation. These cases usually have some connection between the source area and the accumulation area, which is often shallow. This “canal” is eventually identified as an area of atrophic RPE, telangiectasias, distal retinal ischemia, and even perivascular deposits or bony spicules (pseudoretinitis pigmentosa). Chronic cases can also evolve with retinal cysts, cystoid macular edema, and subretinal neovascular membranes8.
ASSOCIATION WITH SYSTEMIC DISEASES
Although it is considered an idiopathic disease, some systemic associations have been observed, especially in chronic or recurrent cases. Situations such as pregnancy, end-stage renal disease, systemic lupus erythematosus (SLE))9, transplant patients10, endogenous corticosteroid production dysfunctions11, use of amphetamines12, use of systemic, epidural, and even inhaled corticosteroids are some examples of associations described with CSC.
Diseases that can lead to choriocapillary ischemia by vasospasm or by precipitation of immunocomplexes, such as SLE, polyarteritis nodosa, Goodpasture’s syndrome, Wegener’s granulomatosis, accelerated hypertension, toxemia of pregnancy, disseminated intravascular coagulation, and idiopathic thrombocytopenic purpura13, can also damage the RPE, leading to RPE and sensorineural retinal detachment.
Some authors have described associations between CSC and gastroesophageal reflux, suggesting a possible relationship with Helicobacter pylori that has yet to be properly clarified14. An association with obstructive sleep apnea has also been described15.
IMAGING TECHNIQUES
FA
The classic pattern of the FA exam presents one or more points of contrast extravasation in the posterior pole at the level of the RPE, slowly filling the serous detachment without going beyond its edges in the course of the exam. Approximately 10% of cases show a “chimney smoke” pattern, in which the extravasation diffuses superiorly and laterally, taking on an “umbrella” or “mushroom” appearance at the upper limits of the detachment (Figure 2A). In only 10% of cases, the extravasation occurs in the fovea. However, most extravasations are concentrated within 1 mm of the fovea and are rare beyond this distance. Therefore, when not visualized, the site is most likely at the upper limit of the detachment or has already healed.
INDOCYANINE GREEN
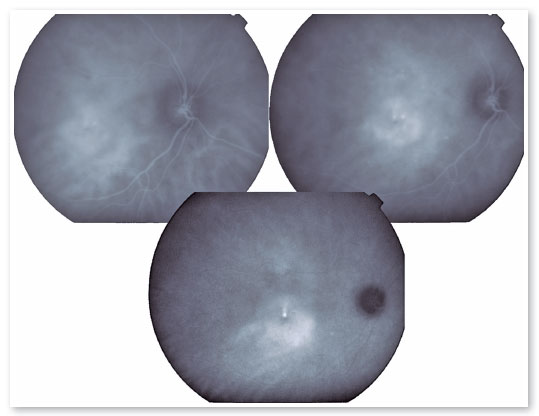
The presence of hyperpermeability in the innermost portions of the choroid is the most frequent finding of the FA examination and is best visualized in the intermediate stages. In the later stages, diffusion of the contrast occurs centrifugally from the initial focus. Hyperpermeability foci also occur in apparently normal areas and do not correspond to the extravasation points identified on FA. Areas of venous engorgement and slowed flow were also identified, which, although nonspecific, contributed to some authors suggesting that CSC may be a less localized pathology than previously thought (Figure 3).
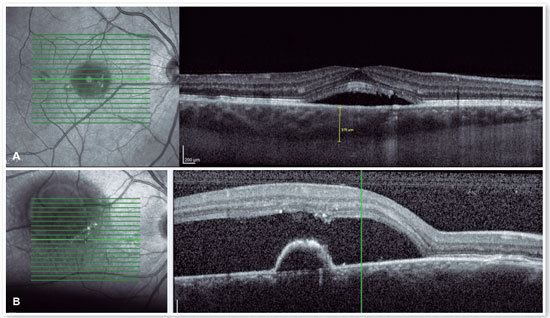
OCT
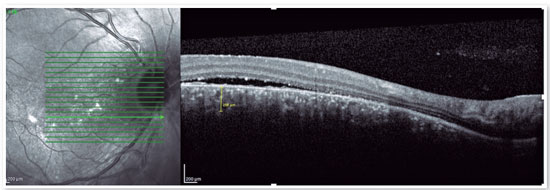
The advent of OCT has brought a more objective way to quantify the size of both RPE and neurosensory retinal detachments, allowing more accurate monitoring of the clinical course of the disease. In addition to accurately assessing the location and amount of subretinal fluid and the location and size of pigment epithelial detachment, OCT also assesses the thickness of the choroid (Figures 4A and B), which is frequently altered in CSC to the point that it is considered a disease on the pachychoroid spectrum16.
NATURAL HISTORY OF THE DISEASE
Approximately 3 months after the onset of symptoms, the serous detachment is spontaneously reabsorbed, leaving only a scar on the RPE, usually larger than the point of extravasation. The patient recovers the previous vision in most cases (more than 90%). Only 5% of the cases progress to severe, permanent visual loss17.
About 30%–50% of patients will develop new episodes, 10% will have three or more recurrences, and 40% may have CSC in the contralateral eye18,19. In 50% of the patients, recurrence occurs in the first year, while in the rest, it occurs up to 10 years or more. In addition, some patients develop chronic detachments involving the macular area, subretinal neovascular membrane, RPE atrophy, or macular cystic degeneration with severe loss of visual acuity. Poor prognostic factors include large RPE detachments, recurrences, fibrin deposits, multiple extravasation points, and “channels” of RPE atrophy.
DIFFERENTIAL DIAGNOSIS
Any pathology leading to serous detachment of the sensorineural retina should be included in the differential diagnosis of CSC, especially those involving the posterior pole.
Inflammatory diseases, such as presumed histoplasmosis syndrome, Harada’s disease, posterior scleritis, sympathetic ophthalmia, and uveal effusion syndrome, should be considered, as should tumors, including melanoma, osteoma, hemangioma (Figure 5), choroidal metastasis, and leukemic infiltrates.
Collagen diseases, including SLE, polyarteritis nodosa, scleroderma, dermatomyositis, and relapsing polychondritis, can cause fibrinoid necrosis of the choroid vessels. In addition, malignant hypertension, toxemia of pregnancy, and disseminated intravascular coagulation due to choriocapillary occlusion can lead to serous detachment.
Cases of optic nerve pit can start with a serous macular detachment, and angiography can help because it shows no point of extravasation. OCT confirms the diagnosis easily.
In patients older than 50 years, especially in chronic cases of CSC, in which there are multiple RPE changes and eventually the development of subretinal neovascular membrane, a differential diagnosis with age-related macular degeneration (AMD) should be made. Indocyanine green becomes useful because it shows early multiple hyperfluorescence that fades with time, whereas in AMD, it shows hyperfluorescence that remains until later stages. Currently, OCT examination and angiography fully confirm the diagnosis of neovascular membrane.
FA and indocyanine green aid in diagnosing polypoidal choroidal vasculopathy by clearly identifying the vascular change. OCT can also provide consistent diagnostic clues, such as broad pigment epithelial detachment or double hump-shaped RPE detachment.
TREATMENT
Considering the spontaneous resolution in most cases, it is recommended that only those patients who are not hindered in their work activity by the visual alteration be clinically observed during the first 3 months of evolution. In addition, reducing the use of any topical, oral, or nasal corticosteroids, among others, should be recommended.
Also, patients with type A personalities, who are usually very stressed, should be advised to change their lifestyle, exercise regularly, and even seek psychological help.
CLINICAL TREATMENT
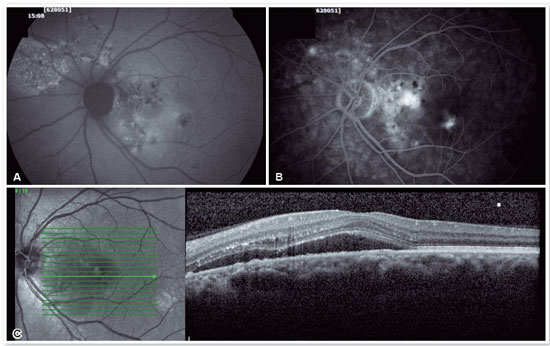
For cases that do not resolve spontaneously or become chronic, it is possible to use medications that inhibit the production of mineralocorticoids or anticorticosteroids, such as spironolactone20,21 and finasteride22, among others. Sleep modulators such as melatonin, beta-blockers to lower stress levels, aspirin, and even rifampin have all been used with controversial results. Intravitreal injections of antivascular endothelial growth factor have not proven effective in treating CSC23 but should be used in cases of complications, such as the appearance of subretinal neovascular membrane (Figures 6 and 7) or polypoidal choroidal vasculopathy.
LASER THERMAL THERAPY
Treatment with photocoagulation is considered only in cases where the leak point is distant from the fovea or when the patient has had CSC in the contralateral eye with poor evolution or recurrence in the eye that had significant visual loss in a previous episode. In chronic cases with large inferior serous detachments or diffuse RPE changes, treatment should also be considered.
After laser application, simple cases take 2 weeks to reabsorb the subretinal fluid, while more complicated ones can take up to 6 weeks.
Possible complications of laser application near the foveal area, such as persistent scotoma, secondary neovascular membrane, and progressive enlargement of the RPE scar, should always be discussed with the patient before application.
The photocoagulation technique should use parameters such as shots of short duration, small target size, and intensity sufficient only to cause a discrete, light stain on the RPE.
TREATMENT WITH RED SHORT-PULSED LASER
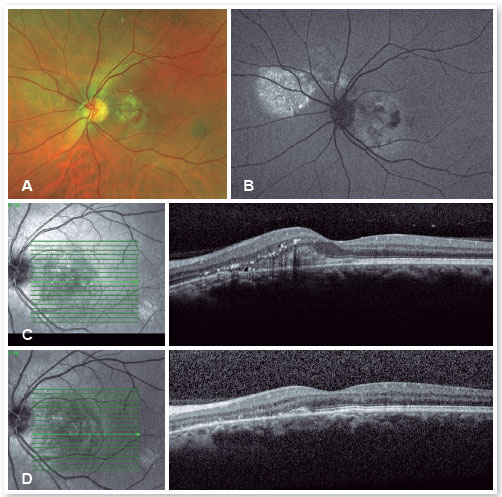
The red short-pulsed laser has shown great results, even in cases with leaks very close to the fovea. The justification for this type of treatment is based on a very light, almost invisible, 10-msec burn that only hits the RPE without affecting the overlying retinal layers, thus decreasing the risk of scotomas and visual loss. Figures 8 and 9 show a case with a chronic juxtafoveal leak that had an excellent result with this type of treatment.

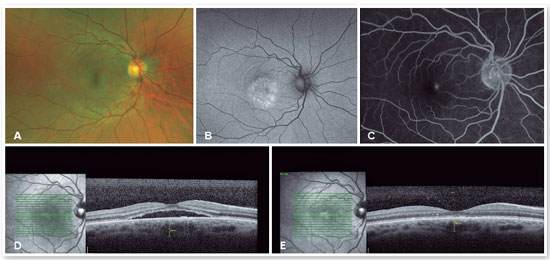
MICROPULSE LASER TREATMENT
The micropulse laser is a good alternative for treating leaky CSC in the foveal avascular zone because it does not cause photoreceptor injury. The micropulse laser releases energy in envelopes, decreasing the heating of adjacent retinal structures, and has a therapeutic effect only on the RPE. Multiple energy envelopes released on the diseased RPE promote restoration of the outer blood-retinal barrier, ultimately leading to subretinal fluid absorption into the CSC. On the other hand, this type of treatment does not act on the choroidal dysfunction and is therefore not always effective24.
TREATMENT WITH PHOTODYNAMIC THERAPY (PDT)
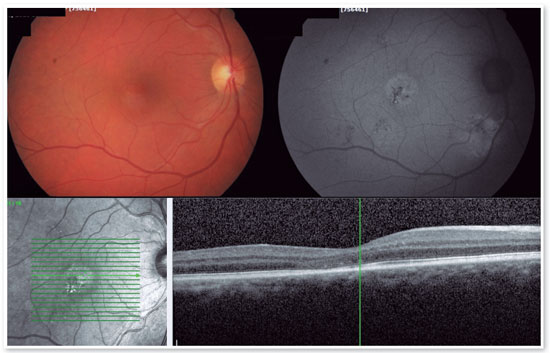
For cases of chronic CSC in which the patient’s central vision is at risk, PDT is an excellent treatment. PDT, which has been used with great results for some time, involves administering an injection of intravenous contrast called verteporfin, which accumulates in the choroid to be treated and absorbs light from a nonthermal infrared laser (690 nm) that promotes a decrease in local choroidal circulation and acts by strengthening the external blood–retinal barrier. As a result, with treatment, there is a decrease in the thickness of the choroid, a decrease in the choroidal circulation, and a consequent absence of fluid leakage into the subretinal space, solving the problem (Figure 10).
Because of the risk of choroidal ischemia, the standard treatment for CSC is half-fluence PDT (half the power in the laser) or a half-dose of verteporfin25. There is no study defining the best alternative, but from personal experience we believe that half fluence PDT is more effective.
REFERENCES
1. Klais CM, Ober MD, Ciardella AP et al. Central serous chorioretinopathy. In: Ryan SJ, editor. Retina, vol. 2. Filadélfia: Elsevier; 2006. p. 1137-9.
2. Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112(8):1057-62.
3. Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63-8.
4. Warren JB, Loi RK, Coughlan ML. Involvement of nitric oxide synthase in the delayed vasodilator response to ultraviolet light irradiation of rat skin in vivo. Br J Pharmacol. 1993;109(3):802-6.
5. Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203-13.
6. Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070-9; discussion 2079-80.
7. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91(12):1554-72.
8. Schatz H, Osterloh MD, McDonald HR, Johnson RN. Development of retinal vascular leakage and cystoid macular oedema secondary to central serous chorioretinopathy. Br J Ophthalmol. 1993;77(11):744-6.
9. Eckstein MB, Spalton DJ, Holder G. Visual loss from central serous retinopathy in systemic lupus erythematosus. Br J Ophthalmol. 1993;77(9):607-9.
10. Fawzi AA, Holland GN, Kreiger AE, Heckenlively JR, Arroyo JG, Cunningham Jr ET. Central serous chorioretinopathy after solid organ transplantation. Ophthalmology. 2006;113(5):805-13.e5.
11. Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110(4):698-703.
12. Hassan L, Carvalho C, Yannuzzi LA, Iida T, Negrão S. Central serous chorioretinopathy in a patient using methylenedioxymethamphetamine (MDMA) or “ecstasy”. Retina. 2001;21(5):559-61.
13. Klais CM, Ober MD, Ciardella AP et al. Central serous chorioretinopathy. In: Ryan SJ, Schachat AP, editores. Retina, vol. 2. Filadélfia: Elsevier; 2006. p. 1150-53.
14. Cotticelli L, Borrelli M, D’Alessio AC, Menzione M, Villani A, Piccolo G, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16(2):274-8.
15. Wu CY, Riangwiwat T, Rattanawong P, Nesmith BLW, Deobhakta A. Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis. Retina. 2018;38(9):1642-51.
16. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103-26.
17. Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91(4):247-50.
18. Donald J, Gass M. Pathogenesis of disciform detachment of the neuroepithelium. I. General concepts and classification. Am J Ophthalmol. 1967;63(3):573-85.
19. Donald J, Gass M. Pathogenesis of disciform detachment of the neuroepithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967;63(3):587-615.
20. Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1985-91.
21. Isaac DLC, Lando L, Silva HM, Avila MP. A bullous variant of central serous chorioretinopathy treated with oral spironolactone. Arq Bras Oftalmol. 2022:S0004-27492022005004216.
22. Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina. 2011;31(4):766-71.
23. Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond). 2013;27(12):1339-46.
24. Yadav NK, Jayadev C, Mohan A, Vijayan P, Battu R, Dabir S, et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye (Lond). 2015;29(2):258-64.
25. Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149(2):307-15.e2.

Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
January 29, 2023.
Accepted on:
February 2, 2023.