Alanderson Passos Fernandes Castro1; Julliana Ferrari Libório Castro2; Wagner Naves1; Camila Azevedo1; Carolina Ramos Mosena de Angeloni1
DOI: 10.17545/eOftalmo/2022.0021
ABSTRACT
Glaucoma is a prevalent optic neuropathy that is characterized by the presence of increased optic disc cupping; however, other ophthalmologic pathologies may also be indicated by similar complications. Therefore, correct and early diagnosis of the etiology of changes in the eye is extremely important in the treatment of patients, especially in cases of potentially progressive and life-threatening diseases, including pituitary neoplasms. Herein, we report a case of misdiagnosis of glaucoma in a patient with a parachiasmatic intracranial expansive lesion, and briefly discuss some aspects that can help in the differential diagnosis of these conditions.
Keywords: Pituitary neoplasms; Glaucoma; Differential diagnosis.
RESUMO
Apesar de o glaucoma ser uma neuropatia óptica bastante prevalente e cursar com o achado característico de escavação do disco óptico aumentada, outras patologias oftalmológicas também podem cursar com quadro semelhante. Dessa forma, o diagnóstico correto e precoce da etiologia da alteração ocular é de extrema importância no tratamento dos pacientes, principalmente em casos de doenças potencialmente progressivas e ameaçadoras à vida, como em neoplasias hipofisárias. O presente artigo busca relatar um caso de equívoco no diagnóstico de glaucoma em paciente com lesão expansiva intracraniana paraquiasmática e realizar uma breve discussão sobre aspectos que auxiliam no diagnóstico diferencial dessas condições.
Palavras-chave: Neoplasias hipofisárias; Glaucoma; Diagnóstico Diferencial.
INTRODUCTION
Glaucoma is a multifactorial degenerative optic neuropathy. It is the second highest cause of blindness, affecting an estimated 76 million people worldwide in 2020, and this number is estimated to increase to 111.8 million patients by 20401. Glaucomatous optic neuropathy (GON) is the most common cause of enlarged optic disc cupping, characterized by loss of neuroretinal rim, deep cupping, optic disc hemorrhages, peripapillary chorioretinal atrophy, decreased retinal arteriole diameter, diffuse or localized loss of nerve fiber layer (NFL), and typical changes in the visual field (VF)2,3. However, in up to 20% of cases, the finding of increased cupping may be attributable to other pathologies2,3.
Regarding non-GONs that present with this finding, anterior ischemic optic neuropathy, hereditary neuropathies, and compressive injuries should be considered4. The latter includes sellar tumors which, through the compression of the anterior visual pathway, cause an increase in cupping prior to visual loss, as well as arcuate scotomas on VC examination, which could be confused with GON3,5-7.
Among pituitary tumors, 50%-80% are adenomas; these are very common benign neuroendocrine tumors and correspond to 15.5% of all tumors of the central nervous system (CNS)8,9. They can be classified by size as macroadenomas (≥10 mm) or microadenomas (<10 mm), and as functional or non-functional, based on endocrine activity8-11. The incidence of adenomas is 3.4 cases per 100,000 inhabitants per year, with a bimodal peak between 25-45 years and 60-70 years9. Nonfunctioning tumors represent 33% of pituitary adenomas and, among those, macroadenomas are the most common, representing 75% of incidental diagnoses9,10. Notably other lesions can affect the chiasmatic region, including parasellar meningioma, craniopharyngioma, and internal carotid aneurysm12.
Visual impairment may occur as a result of a chronic increase in intracranial pressure, more specifically owing to compression or tumor invasion in the optic pathway11. Visual field defects-typically bitemporal hemianopsia-and low vision occur in 9%-32% and 4%-16% patients, respectively1. Other manifestations include headache, cranial neuropathy with and without ophthalmoplegia, ptosis, and endocrinopathy11.
Regarding diagnosis, magnetic resonance imaging (MRI) of the sellar region is the gold standard method for evaluating pituitary adenomas. The presence of visual damage, such as a visual field defect, is indicative of surgical treatment. However, in 10%-20% of cases, the visual field does not improve after surgery13.
The treatment of choice is transsphenoidal resection, which can be performed through the endoscopic or microscopic approach, whereas the transcranial approach is indicated for large tumors that extend to the middle fossa and cavernous sinus. Nonfunctioning adenoma has a recurrence rate of approximately 20%-30%; thus, an MRI is performed every 3-6 months after surgery, annually for 5 years, and biannually for an additional 6 years. The two most significant factors for recurrence are extension into the cavernous sinus and the presence of adenoma persisting after surgery. Postoperative radiotherapy can help reduce recurrences from 40% to 10%; however, the associated adverse effects must be considered, including pituitary hormone deficiency, cerebrovascular event, optic nerve damage, and brain tumors9. Other options are drug suppression and radiotherapy8.
We aim to report a case of pituitary macroadenoma with significant visual function alteration simulating normal pressure glaucoma (NPG), with further discussion on the differential diagnosis between the two pathologies.
CASE REPORT
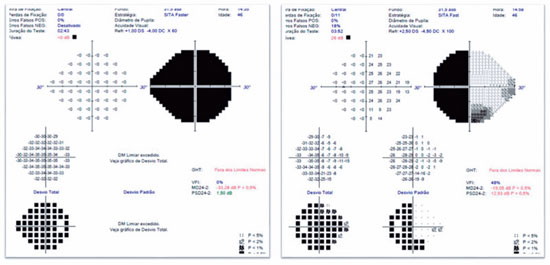
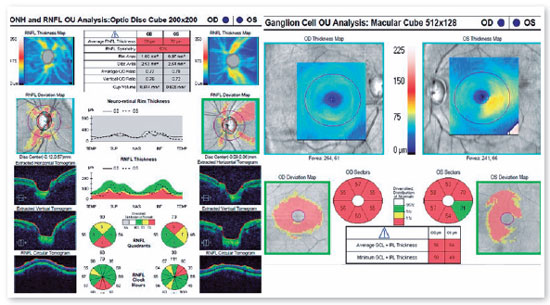
A 46-years-old man, complained of visual loss in the right eye (OD) beginning 8 months before the study period. He was diagnosed with glaucoma at another Healthcare center, prescribed with a combination of 0.2% brimonidine and 0.5% timolol for 6 months, and presented with iridotomy in both eyes (OU) 4 months previously. Upon examination, corrected visual acuity (AVcc) in the OD was light perception (+1.00 -4.00 × 60) and in the left eye (OS) was 20/50 (+2.50 -4.50 × 100). Reduced direct photomotor reflex was observed in the OD in addition to relative afferent pupillary defect, biomicroscopy with iridotomy at 1 hour in OU, intraocular pressure (IOP) of 12/12 mmHg and funduscopy with increased cupping (0.8 × 0.8) in OU, as well as total pallor of the papilla and macula without changes in OU. Owing to the fact that the IOP was within the normal range and the presence of a previous acute glaucoma crisis was not indicated, the hypothesis of changes in the optic disc as a result of neurological or cardiovascular etiologies was considered. Cranial MRI, carotid Doppler, VF 30.2, optical coherence tomography (OCT), and retinography were prescribed. He returned with VF showing amaurosis on the right and loss of the temporal hemifield and lower nasal part on the left (Figure 1); retinography with bilateral optic atrophy and signs of grade 1 hypertensive retinopathy (Figure 2); OCT of the OD with cupping of 0.77 × 0.76 and loss of superior and inferior NFL, and OS with cupping of 0.78 × 0.72 and loss of superior and temporal NFL, as well as retinal ganglion cells with bilateral losses (Figure 3). Carotid Doppler with left carotid intima-media thickening, without stenosing plaques. Finally, MRI identified a parachiasmatic intracranial expansive lesion compressing the optic chiasm (Figure 4). The patient was then referred to the neurosurgery service.

DISCUSSION
Although optic neuropathy is most commonly associated with increased optic disc cupping, this finding is not a pathognomonic sign of GON, thereby highlighting the need to establish a differential diagnosis with other neuropathies. One such neuropathy, compressive optic neuropathy (CON), has been reported in this case2.3.
In many cases of CON, atrophy of the optic nerve is the only finding on funduscopic examination. Furthermore, a diagnostic confusion exists regarding NPG; one study observed a case series of 6.5% of patients previously diagnosed with NPG who had a compressive lesion14.
Another study, conducted in 2011, evaluated 501 patients with sellar tumors and identified an optic disc with a glaucomatous appearance significantly more frequently than in the control group. This finding was more closely associated with tumor location and size, i.e., the larger the tumor and the closer the internal opening of the optic canal, the more frequent the glaucomatous appearance. Among the hypotheses examined, sellar tumors located close to the ON are believed to block the passage of cerebrospinal fluid to the orbit through the ON channel. This would result in a collapse of the retrobulbar cerebrospinal fluid space, causing an increase in pressure gradient across the cribriform lamina. This situation would mimic what occurs in the case of normal retrobulbar cerebrospinal fluid; however, with increased IOP. Corroborating this hypothesis, recent studies have suggested that some patients with NPG may have low cerebrospinal fluid pressure15.
However, although initially, the case reported was similar to that of NPG, glaucomatous patients have loss of neuroretinal rim and deep cupping. The rim is preserved in CON, albeit paler, which can be observed when evaluating the paler rim. on retinography7,14. Corroborating this fact, a study from 2016 showed that in early stages, eyes with CON have greater rim pallor than those with GON, despite having the same degree of nerve fiber involvement, reflecting different histopathological characteristics in relation to glial architecture in each pathology. However, in moderate to advanced stages, this relationship would not be maintained14.
Glaucomatous cavitation is understood to be associated with the death of ganglion cells, and is referred to as laminar or deep. Glaucomatous cupping is believed to have a greater anterior laminar depth than non-glaucomatous cupping5. Based on this, a 2020 study used the measurement of the minimum rim width at the opening of the Bruch’s membrane (BMO-MRW)-a true anatomic edge of the neuroretinal rim-for quantitative assessment of cupping. The results demonstrated that patients with GON showed significantly lower values than those with non-GON5. When directly comparing CON and GON, another study, conducted in 2020, demonstrated that the ratio of nasal BMO-MRW to peripapillary NFL (N-BMO-MRW/pRNFL) with values ≥4.24 showed diagnostic sensitivity and specificity of 84% and 72%., respectively. However, in patients with non-GON, MRW is preserved, despite the diffuse loss of NFL16. Although this assessment alone does not allow a differential diagnosis, it contributes to considering differential diagnoses of non-glaucomatous etiology, and to performing a more accurate clinical examination and thereby assessing the need for additional tests. Notably, this measurement does not specifically differentiate the type of non-GON5.
Another parameter used to differentiate GON and CON is the thickness of the macular ganglion cell layer, especially in the nasal subfields, which leads to a reduction in thickness prior to the appearance of changes in visual field in cases of CON7,17. Furthermore, it is a well-established fact that CON affects the NFL more temporally, in particular, and also nasally, whereas GON is more likely to affect the upper and lower regions of the NFL3,7,17. When compared to early glaucomas, loss of papillomacular bundle fibers is more common in non-GON, regardless of etiology17. However, when comparing advanced glaucomas, this differentiation is lost owing to an increased greater involvement of the NFL7.
Finally, some authors cited factors as indicative of non-GON and the need for neurological investigation. These factors include the following: young age (<50 years), low visual acuity (<20/40), vertical visual field defects in relation to the midline, pallor of the neuroretinal rim, asymmetrical loss of color vision, and relative afferent pupillary defect. Since the patient in the case reported had at least five of these factors, the initial diagnosis of glaucoma was considered as opposed to a non-glaucomatous etiology4,7.
Although glaucoma is a much more frequent neuropathy than CON, a detailed, critical clinical examination should always be performed, paying attention to the often subtle signs of non-glaucomatous etiology, such as those presented in this article. This will enable a more accurate diagnosis of the patient’s real condition, as well as adequate treatment, resulting in a more favorable visual prognosis with the possibility of early treatment.
REFERENCES
1. Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus. 2020;12(11): e11686.
2. Kosior-Jarecka E, Wróbel-Dudzińska D, Pietura R, Pankowska A, Szczuka B, Żarnowska I, et al. Results of Neuroimaging in Patients with Atypical Normal-Tension Glaucoma. Biomed Res Int. 2020 Aug 18;2020:9093206.
3. Lee J, Kim JS, Lee HJ, Kim SJ, Kim YK, Park KH, et al. Discriminating glaucomatous and compressive optic neuropathy on spectral-domain optical coherence tomography with deep learning classifier. Br J Ophthalmol. 2020;104(12):1717-23.
4. Dias DT, Ushida M, Battistella R, Dorairaj S, Prata TS. Neurophthalmological conditions mimicking glaucomatous optic neuropathy: Analysis of the most common causes of misdiagnosis. BMC Ophthalmology. 2017;17(1):2.
5. Leaney JC, Nguyen V, Miranda E, Barnett Y, Ahmad K, Wong S, et al. Bruch’s Membrane Opening Minimum Rim Width Provides Objective Differentiation between Glaucoma and Nonglaucomatous Optic Neuropathies. Am J Ophthalmol. 2020 Oct;218:164-72.
6. Mimouni M, Stiebel-Kalish H, Serov I, Chodick G, Zbedat M, Gaton DD. Optical Coherence Tomography May Help Distinguish Glaucoma from Suprasellar Tumor-Associated Optic Disc. J Ophthalmol. 2019 Oct 29;2019:3564809.
7. Yum HR, Park SH, Park HYL, Shin SY. Macular ganglion cell analysis determined by Cirrus HD optical coherence tomography for early detecting chiasmal compression. PLoS One. 2016;11(4): e0153064.
8. Kim TG, Jin KH, Kang J. Clinical characteristics and ophthalmologic findings of pituitary adenoma in Korean patients. Int Ophthalmol. 2019;39(1):21-31.
9. Mercado M, Melgar V, Salame L, Cuenca D. Clinically non-functioning pituitary adenomas: Pathogenic, diagnostic and therapeutic aspects. Endocrinol Diabetes Nutr. 2017;64(7):384-395.
10. Iglesias P, Arcano K, Triviño V, García-sancho P, Díez JJ, Villabona C, et al. Prevalence, Clinical Features, and Natural History of Incidental Clinically Non-Functioning Pituitary Adenomas. Horm Metab Res. 2017;49(9):654-659.
11. Uche NJ, Uche EO, Eze BI, Amuta DS, Guga DA, Okpara S. Neurophthalmic manifestations of giant sellar tumors in adults: Relationship between endocrine status and visual acuity profile pre and postsurgical treatment: A prospective cohort study. Niger J Clin Pract. 2020;23(11):1500-1506
12. American Academy of Ophthalmology. 2020-2021 Basic and Clinical Science Course, section 05: Neuro-ophthalmology. San Francisco: American Academy of Ophthalmology, 2020.
13. Hisanaga S, Kakeda S, Yamamoto J, Watanabe K, Moriya J, Nagata T, et al. Pituitary Macroadenoma and Visual Impairment: Postoperative Outcome Prediction with Contrast-Enhanced FIESTA. AJNR Am J Neuroradiol. 2017;38(11):2067-2072.
14. Nakano E, Hata M, Oishi A, Miyamoto K, Uji A, Fujimoto M, et al. Quantitative comparison of disc rim color in optic nerve atrophy of compressive optic neuropathy and glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1609-16.
15. Qu Y, Wang YX, Xu L, Zhang L, Zhang J, Zhang J, et al. Glaucoma-like optic neuropathy in patients with intracranial tumours. Acta Ophthalmol. 2011;89(5):e428-33.
16. Braga J, Soares R, Loureiro M, Ribeiro L, Meira D. Bruch’s Membrane Opening Minimum Rim Width in the Differential Diagnosis of Optic Neuropathies. Neuroophthalmology. 2019; 44(2):76-88.
17. Xiao H, Liu X, Lian P, Liao LL, Zhong YM. Different damage patterns of retinal nerve fiber layer and ganglion cell-inner plexiform layer between early glaucoma and non-glaucomatous optic neuropathy. Int J Ophthalmol. 2020;13(6):893-901.
AUTHOR’S INFORMATION



Funding: No specific financial support was available for this study
Conflict of interest: None of the authors have any potential conflict of interest to disclose
Received on:
July 11, 2022.
Accepted on:
October 11, 2022.