Márcia Beatriz Tartarella1; João Borges Fortes Filho2
DOI: 10.17545/e-oftalmo.cbo/2016.74
ABSTRACT
This study discusses current aspects regarding retinopathy of prematurity (ROP), a clinical disorder that leads to the highest number of children in the world becoming blind. This emphasizes the importance of screening for the disease in all preterm infants with a very low birth weight, identifying retinopathy at a proper time, and treating it using laser at about 37 weeks post-conception while there is still a chance for treatment, which should preferably be conducted during the child stay at the Neonatology Center. The first ophthalmic examination should be performed using binocular indirect ophthalmoscopy, with dilated pupils, between 4 and 6 weeks after birth in all newborns weighing 1,500 g or less and/or at the gestational age of 32 weeks or less, as proposed by the Brazilian Guidelines for ROP Screening and Detection. The follow-up of children with or without retinopathy should be conducted periodically until vascularization of Zone 3 temporal retina is normalized, and this normalization should be maintained during the first years of life for the prevention of amblyopia and strabismus and for the correction of refractive errors related to prematurity.
Keywords: Retinopathy of Prematurity; Blindness; Prevention & control; Prevalence
RESUMO
Este artigo aborda aspectos atuais sobre a retinopatia da prematuridade (ROP), entidade clínica responsável pela maior quantidade de crianças cegas em todo o mundo nos dias de hoje. Procura chamar a atenção para a importância da triagem na busca da doença em todos os prematuros de muito baixo peso e a necessidade de que a retinopatia seja identificada no momento adequado e tratada pelo laser ao redor da 37ª semana de idade pós-concepção, quando ainda existe chance para o tratamento que deverá ser feito, preferencialmente, durante o tempo de permanência da criança no Centro de Neonatologia. O exame oftalmológico inicial deve ser realizado sob oftalmoscopia binocular indireta e dilatação das pupilas, entre a 4a e a 6a semana de vida, em todos os recém-nascidos com peso igual ou menor do que 1500 gramas e/ou com idade gestacional igual ou inferior a 32 semanas conforme proposto pelas Diretrizes Brasileiras de Triagem e Detecção da ROP. O acompanhamento das crianças com ou sem retinopatia deverá seguir periodicamente até a normalização da vascularização da retina temporal na zona III e deverá ser mantido pelos primeiros anos de vida para a prevenção da ambliopia e do estrabismo e para a correção das altas ametropias relacionadas com a prematuridade.
Palavras-chave: Retinopatia da prematuridade: Cegueira: Prevenção & controle: Prevalência
INTRODUCTION, DISEASE CHARACTERISTICS, AND LITERATURE REVIEW
Described more than 50 years ago, retinopathy of prematurity (ROP) has become one of the main causes of blindness during childhood worldwide. ROP, in its natural evolution, incurs high social and financial costs for the whole community because it causes irreversible vision damage, impairing the cognitive as well as psychomotor development of the affected child. 1,2,3
ROP was first described by Terry in 1942 as retrolental fibroplasia (FRL). He identified an abnormal growth of the fibroblastic tissue and blood vessels juxtaposed posteriorly to the lens, causing bilateral blindness in children born prematurely. Between 1942 and 1945, Terry reported a total of 117 cases of blindness among surviving preterm infants.45 Ten years later, FRL was the major cause of blindness among children; Silverman estimated that between 1943 and 1953, more than 7,000 children born prematurely were blind due to this disease in the United States. 6
As new knowledge was gathered about the pathogenesis of this disease, the term FRL was gradually revised to ROP by Health in 1951 to aptly describe the condition. 7
The relationship between ROP and the use of oxygen, which is vital for the survival of preterm infants, was evidenced by Campbell in Australia in 1951 8 and by Crosse and Evans in England in 1952.9 These studies suggested that the indiscriminate use of oxygen right after a premature birth was related to the onset of ROP, producing an immediate effect all over the world. Between 1951 and 1960, the use of postnatal oxygen was a major restriction, leading to the false impression that ROP was under control. This period presented a reduction in cases of blindness caused by ROP, but it also coincided with a higher mortality rate or increased comorbidities among surviving preterm infants. In 1973, Cross estimated that for each case wherein blindness caused by ROP was prevented, there were 16 cases of death among preterm infants in the United States. 10
The period between ROP identification and the early 1960s became known as the “first ROP epidemic.” From 1960 to 1970, the use of oxygen increased in Neonatal Intensive Care Units (NICUs), and again, many cases of ROP were reported as well as an important increase in the survival of preterm infants was noted. This period was known as “the second ROP epidemic.” Since the 1980s, a “third ROP epidemic” has been identified by the international scientific community, with ROP as the major cause of childhood blindness in all countries with growing economies, due to a considerable increase in the survival of very low birth weight (VLBW) preterm infants (VLBW ≤ 1,500 g), particularly in the survival of extremely low birth weight (ELBW) preterm infants (ELBW ≤ 1,000 g). 11,12,13
ROP EPIDEMIOLOGY IN BRAZIL
ROP has been considered one of the major causes of childhood blindness in countries with growing economies, including Brazil. 11,13 In recent years, with more and more hospitals offering better care for high-risk pregnancies and improvements in the care quality, an increase has been reported in the survival rate of ELBW preterm infants, from 8% to approximately 35%, in most developing countries.13
Many institutional studies have been conducted, allowing a better understanding of ROP in Brazil. In 1995, Moraes et al. prospectively analyzed a population of 1,342 preterm infants with BW ≤ 2,100 g and gestational age (GA) at birth ≤ 37 weeks, born in São Paulo, and detected ROP in 28% of the cohort; 80% of ROP at any stage occurred in infants with BW ≤ 1,000 g, and 42% of ROP occurred in infants with GA at birth ≤ 29 weeks. 14 In a study published in 2007 and conducted in Joinville, Santa Catarina, South Brazil, Bonotto et al. analyzed data on 286 preterm infants with GA at birth ≤ 37 weeks and reported 20% prevalence of ROP at any stage.15 In Rio de Janeiro in 2009, Schumann et al. reported data on the occurrence of ROP: 53.4% among 73 patients had a BW ≤ 1,500 g and/or GA at birth ≤ 32 weeks. 16 A study conducted by Pinheiro et al. in Natal, Rio Grande do Norte, Northeast Brazil, analyzed data on 663 preterm infants with BW ≤ 1,500 g and/or GA at birth ≤ 36 weeks and reported an ROP incidence of 62.4% between 2004 and 2006. — Filho et al. in Porto Alegre, Rio Grande do Sul, South Brazil, published several prospective studies that included and collected data on patients according to the Brazilian Guidelines for ROP Examination and Treatment, published in 2007. 18 The prevalence of ROP at any stage from 2002 in infants with BW ≤ 1,500 g and/or GA at birth ≤ 32 weeks (VLBW) was reported to be around 25%, whereas that of severe ROP requiring treatment (threshold ROP or Type 1 prethreshold ROP) was 5%. When considering infants with BW and GA below 1,000 g and 28 weeks (VLBW), the occurrence of ROP was much higher, reaching 45% and 17%, respectively, for ROP at any stage and for severe ROP requiring treatment.19,20,21,22 These important variations in the prevalence of ROP observed in different studies published in Brazil show great regional differences among the Brazilian population as well as considerable differences in the general neonatal care provided in the country.
In Brazil, estimates indicate that every year more than 15,000 surviving preterm infants are in the risk group for ROP (BW ≤ 1,500 g and/or GA at birth ≤ 32 weeks), leading to a prediction of blindness caused by ROP in 500 to 1,500 patients per year in the country. 22,23
PATHOGENESIS OF ROP
The pathogenesis of ROP is still not entirely clear. Since 1942, when the disease was identified, and for many years after that, it was thought that the high levels of oxygen administered to premature infants would have a crucial role and be the only cause of ROP. In prospective and controlled clinical studies, Patz et al, 24 clearly demonstrated the cause-and-effect relationship between oxygen therapy in mechanical ventilation and the onset of ROP; however, the disease appeared even after careful control over the administration of oxygen in hospital nurseries. The level of safety when using oxygen therapy in preterm infants has not been sufficiently demonstrated, although it has been widely demonstrated that the continuous monitoring of oxygen reduces the incidence of ROP. These facts characterize ROP as a multifactorial disease. 25
During the embryonic development of the eye, the vascularization of the nasal retina is completed around 32 weeks post-conception, whereas that of the temporal retina is completed around 40 weeks post-conception or soon after full-term birth. Therefore, ROP can occur as a result of an interruption in the process of natural retinal vascularization due to premature birth. 26
VEGF, IGF-I, AND DEVELOPMENT OF ROP
ROP is a vasoproliferative ocular disease secondary to inadequate retinal vascularization, which occurs in preterm infants whose retinas are immature at birth. After a premature birth, the supplemental oxygen given to a newborn leads to a situation of hyperoxia, triggering vasoconstriction, vascular obliteration, peripheral ischemia, and permanent interruption of retinal vascularization. Hyperoxia, if maintained for a longer period, leads to the overproduction of the vascular endothelial growth factor (VEGF), which stimulates unwanted neovascularization of the retina and the appearance of other ROP complications. 26
ROP is a two-stage disease and its onset is associated with VEGF, an oxygen-regulated factor, and with insulin-like growth factor-I (IGF-I), which is not regulated by oxygen.27 In individuals who develop ROP, the growth of peripheral retinal vessels is either slowed down or permanently interrupted after a preterm birth, leaving the peripheral retina avascular and hypoxic (stage 1 of ROP). The proliferative phase of the disease (stage 2 of ROP) is a result of this ischemia. The full extent of the lack of retinal perfusion in the early phase of ROP seems to determine the subsequent degree of neovascularization, that is, the severity of the disease that may lead to retinal detachment and irreversible blindness. 27,28
VEGF is a powerful angiogenic factor required for normal blood vessel growth, but it is also associated with unwanted neovascularization of the retina and iris. In a preterm birth, VEGF expression is reduced. It is believed that this phenomenon is due to hyperoxia to which the newborn is subjected. As the retina matures and becomes hypoxic because of the interrupted vascular growth, VEGF levels progressively increase, producing unwanted retinal neovascularization in some patients (stage 2 of ROP). However, VEGF inhibition at this stage does not completely prevent the retinal neovascularization of ROP, indicating that this is a multifactorial disease. 27
In recent years, many studies have shown the relationship between IGF-I and the onset of ROP. This association was first demonstrated when investigators found that this factor controlled maximal VEGF activation. Hellström et al. demonstrated that very low levels of IGF-I prevented the in vitro activation of protein kinase B, critically involved in maintaining endothelial cell survival. These findings explain how reduced IGF-I levels can cause disease and prevent the normal survival of endothelial cells.27 These observations were confirmed in patients with ROP. Hellström et al. reported that in preterm infants, serum levels of IGF-I immediately after birth were significantly and proportionally lower in those who developed ROP compared with the levels in those who did not. 28 Villegas-Becerril et al. found that in preterm infants with BW ≤ 1,500 g and GA at birth ≤ 32 weeks, the serum levels of IGF-I measured between the first 4-6 weeks after birth were much lower in preterm infants with ROP compared with the levels in those without, and suggested that IGF-I is an indicator of ROP. 29
IGF-I is required for the normal development of retinal vascularization. Low serum levels of IGF-I inhibit the natural vascularization of the retina, whereas high serum IGF-I levels stimulate neovascularization (stage 2 of ROP). Low serum IGF-I levels in the first weeks after a preterm birth are thought to be associated with an increased chance of late ROP development. The period for which a preterm infant has low serum IGF-I levels could be related to the severity of the disease. 30,31
RISK FACTORS FOR THE ONSET OF ROP
Not all preterm infants develop ROP. There are many factors that could trigger the onset of ROP and its serious complications. For many years now, oxygen therapy is no longer considered the only or main risk factor (RF) for the onset of ROP. It is a multifactorial disease, and the use of oxygen is just one of many factors involved in this onset. The influence of other factors, as a whole, has not been clearly defined to date. 32
ROP more frequently affects the smallest and most clinically debilitated preterm infants, but it can also affect preterm infants with a higher BW. It has not yet been sufficiently demonstrated whether the more severe forms of the disease are associated with the therapeutic interventions required to maintain the life of preterm infants or with the severity of the comorbidities associated with prematurity. 33
Many RFs for the onset of ROP have been studied in the last 50 years, and different prenatal and postnatal causes have been proposed to explain the onset of ROP in premature infants: bacterial or viral infection, either primary or transmitted by the mother to the preterm infant; vitamin E, iron, or hormonal deficiencies; anoxia; anemia; and hypercapnia; 34 However, lower gestational age (general immaturity), poor physical condition of the preterm infant, low BW and the extended use of oxygen therapy have been the most consistent causes related to the onset of ROP. 33,34
The prenatal use of steroids and beta-blockers by the infant's mother; the infant's exposure to intense light in hospital nurseries; the use of corticosteroids, indomethacin, surfactants, and erythropoietin by the infant; twin pregnancy; the need for ductus arteriosus treatment; the presence of intracranial hemorrhage; recurrent apnea; blood transfusions; immunosuppression; the concomitant presence of infections; and the use of antibiotics have also been associated with the onset of ROP. 35
The presence of intracranial hemorrhage (ICH) at any stage has been mentioned as a RF for ROP. In 1981, Procianoy et al. reported a significant association between cicatricial ROP and the occurrence of ICH when studying 138 VLBW preterm infants. 36 In 2002, Christiansen reported a significant association between the ICH stage and ROP staging in a cohort of 60 VLBW preterm infants, wherein 17 patients developed ICH stages III and IV and 49 developed ICH stages 0 to II. Sixty-four percent of premature infants who developed advanced ICH stages presented stage 3 of ROP or worse. 37
Prenatal factors that could impact the overall health of patients and lead to the development of ROP have also been studied. They include the use of corticosteroids or beta-blockers in the prepartum period and the maternal age at birth due to the growing incidence of pregnancy among pre-adolescents, particularly in developing countries. 38 The occurrence of preeclampsia in mothers is reported to be a protective factor for preterm infants in terms of the subsequent development of severe ROP. 39
Low weight gain (WG) after a preterm birth as a potential RF for the onset of ROP has been reported in the scientific literature only more recently. The first study to correlate WG in the postnatal period with ROP in a series of patients was published by Wallace et al. in 2000. The authors retrospectively collected postnatal WG data as well as data on 11 other RFs and suggested that a WG below 50% in relation to the BW in the first 6 weeks after birth is an important RF for ROP in its most severe forms (stages 3, 4, or 5). 40 Filho et al. prospectively analyzed low WG in the period after birth and its importance as a predictive factor for ROP. The study, published in 2009, demonstrated the possibility of predicting ROP through ROC curve analysis (in relation to WG sensitivity and specificity) when studying WG progress after the preterm birth. This fact is very important and convenient in the routine of ophthalmologists.41 Several recent studies have also indicated a possible genetic predisposition to ROP in preterm patients. 42
INTERNATIONAL CLASSIFICATION OF ROP
The International Classification of ROP was first developed in 1984 by a group known as the Committee for the Classification of Retinopathy of Prematurity, and it was revised in 1987 and 2005. The International Classification places it as a retinal disease, affecting three established retinal zones, and describes the disease extent according to the involved meridians, represented as clock hours (Figure 1). The changes resulting from ROP progress are classified into five stages as follows:
ROP stages:
• Stage 1: Peripheral retinal ischemia with a demarcation line between the vascularized retina and the ischemic retina;
• Stage 2: Enlargement of the demarcation line and presence of a ridge over the peripheral region of the retina;
• Stage 3: Retinal or extraretinal fibrovascular proliferation in the ridge areas;
• Stage 4A: Extrafoveal retinal detachment;
• Stage 4B: Subtotal retinal detachment involving the fovea; and
• Stage 5: Total retinal detachment, complicated by the proliferation of fibrous or retrolental tissue
Map for the location of disease in retinal zones
The map created in 1984 (Figure 1) shows two concentric circles, with the optic papilla in the center. A third semilunar circle was positioned in the temporal sector. These circles defined three different zones of the optic papilla and those of disease involvement:
• Zone I (posterior pole): defined as a circle with the optic nerve in its center and a radius twice the distance from the center of the optic nerve to the center of the macula in all directions;
• Zone II (equatorial zone): extending from the edge of Zone I to a circle with a radius equal to the distance from the optic nerve to the nasal ora serrata; and
• Zone III (peripheral temporal zone): the residual crescent area external to Zone II in the temporal sector. This zone has late retinal vascularization in preterm infants; therefore, it is the zone more frequently involved in ROP.
Disease extent
It has been stated that the extent of the disease would be measured in clock hours. When looking at the map, the 3-h meridian is located on the nasal side of the right eye and temporal side of the left eye.
Concept of plus disease
A premature infant presents ROP with plus disease when significant dilation and tortuosity of the venous or arterial retinal vessels are identified on examination, from the periphery to the posterior pole (Figure 2). At that moment, discrete vitreous turbidity may occur as well as vascular changes in the iris and difficulty in achieving a good dilation of the pupils may be noted. The clinical importance of these findings is that they indicate ROP progression, with an increased risk of vision loss in the affected infant. The occurrence of plus disease is considered the most important and immediate indicator for the treatment of a patient with ROP. 43,44,45
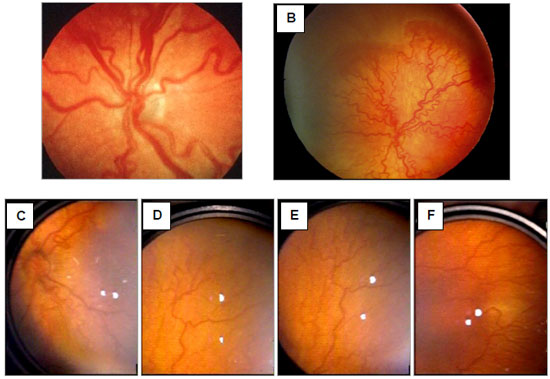
In 2005, new changes were made to the international classification, with the introduction of the concept of rush disease or aggressive posterior ROP, especially observed among ELBW preterm infants. The changes also included the concept of an intermediate level of the plus disease (pre-plus disease) as well as a method to easily estimate the extent of Zone I when performing binocular indirect ophthalmoscopy with a 28-diopter lens. 45 The updated ROP classifications are shown in Table 1.
ROP DIAGNOSIS IN SCREENING EXAMS
Ophthalmologic screening programs for the detection of ROP, with eye exams performed systematically at NICUs in premature infants born with a risk for ROP comprise the best method to diagnose the disease for ensuring a proper treatment before the natural progression to its later stages. The inclusion criteria in the risk groups vary with the country and are based on BW and GA. 46
In Brazil, since 2007, the Screening Guidelines for ROP Detection and Treatment have defined that screening for early detection of ROP should be performed on all preterm infants with BW ≤ 1,500 g and/or GA at birth ≤ 32 weeks. The first eye examination would be performed between 4 and 6 weeks after birth using binocular indirect ophthalmoscopy (BIO) and dilated pupils. The screening could also include all infants with higher BW or higher GA who presented any chance of developing the disease due to their clinical conditions, as requested by the neonatologist in charge. 18
Clinical examination
Eye examination of all patients should always be performed using BIO after dilation of the pupils, with eye drops containing tropicamide 0.5% and phenylephrine 2.5%, instilled 3 times, at 5-min intervals before the examination. Also, 20-, 28-, or 30-diopter lenses can be used. Blepharostats for newborns, especially the Alfonso Eye Speculum (Storz®, USA), and scleral indentation may be used for easy observation of abnormalities in Zone III. Topical anesthesia of the ocular surface with one drop of proxymetacaine hydrochloride 0.5% is advisable.
The Brazilian guidelines indicate that eye exams should begin between 4 and 6 weeks after birth and be repeated according to the findings of the first examination as follows: mature retina (complete vascularization): 6-month follow-up; immature retina (non-complete vascularization) or presence of prethreshold ROP: evaluation every 2 weeks; regression of retinopathy: evaluation every 2 weeks; immature retina in Zone I: weekly exams; Type 2 prethreshold ROP: exams every 3 to 7 days; and Type 1 prethreshold ROP (Zone I, any stage with plus disease; Zone I, stage 3; and Zone II, stage 2 or 3 with plus disease) and threshold ROP: treatment within 72 h of diagnosis. Finally, the guidelines recommend the interruption of exams only after retinal vascularization is complete or the patient presents the corrected GA after 45 weeks, with the absence of prethreshold ROP, or when ROP occurs in a completely regressed form. 18
Complementary examinations that can be useful
In most cases, the diagnosis of ROP is almost entirely based on the identification of the disease through an examination of the infant in the NICU using BIO, a technique that requires much training of the examiner for it to be used on preterm infants. Recently, other methods have been used to complement or facilitate the screening for the disease, including fluorescein angiography, ultrasonography, optical coherence tomography, measurement of the velocity of blood flow in the central artery of the retina through Doppler, and the documentation of the eye examination through RetCam, mobile phones with a camera, and the use of telemedicine.
Fluorescein angiography
It is still a semiological method of very inconvenient use in premature infants, unless it can be performed with RetCam 120 (Massie Research Laboratories, Dublin, CA, USA), which has been especially developed for pediatric ophthalmology. This equipment allows a great view of the fundus in high-angle fields that reach the full 360° of the retinal periphery. Thus, the ischemic and neovascular phenomena that occur in ROP can be fully documented, making it a valuable device to fight blindness caused by ROP. RetCam is a versatile computerized device that is being used worldwide in ROP-related telemedicine programs. RetCam can accept the excitation and block filters required for fluorescein angiography. The 20% fluorescein sodium dye can be injected intravenously at a dose of 0.04 ml/kg of body weight (8 mg/kg), followed by an amount of physiological saline solution, to ensure a suitable amount in the infant's blood stream. Complications that may occur in fluorescein angiography with adults may also occur with preterm infants, and all emergency care needs to be available before this exam is performed in this group of patients. However, studies have already reported that the use of fluorescein angiography is a safe procedure in preterm infants. 47,48
The major advantage of fluorescein angiography is that it provides an easier view of the peripheral vascularization of the retina that may not be clearly identified in the examinations using BIO or RetCam without an intravenous dye. Angiography may be useful to ensure a more precise identification of the posterior pole and macular region in patients with severe ROP in Zone I and to define the total extent of the laser required for a proper treatment, especially with ROP in Zone I or II. It is also useful in the identification of areas without proper photocoagulation in those patients whose plus disease persists for more than 20 days after the initial laser treatment. The persistence of plus disease after this period would be an indication for the requirement of a complementary laser treatment.
Ultrasonography
Ultrasonography, with 10 or 20 MHz probes, has been routinely used for many years in patients with ROP in stages 4 and 5, especially when planning retinal detachment repair surgeries. Through ultrasonography, the conditions of adherence of the detached retinal leaflets in the form of an open or closed funnel can be observed both anteriorly and posteriorly. Vitreous traction and opacification, increased choroidal thickness, and reduction of the anteroposterior length of the eyeball can be very well identified by ultrasound, allowing a prognosis of retina re-application after surgical procedures. 49
It should be noted that the axial length of the eyeballs of infants who present late-stage ROP in week 39 or 40 post-conception is 16 to 17 mm on average, and ultrasound probes reach a focal plane on the retina of an eye whose axial length is 24-30 mm. This leads to a reduction in the ultrasound resolution in retinal or pre-retinal structures in preterm patients. For this reason, many researchers recommend that ultrasound should be used in premature infants only with a liquid chamber interposed between the transducer tip and the cornea so that the plane of the retina remains within the probe's field of higher resolution, around 24-30 mm. This liquid chamber could be built using one finger of a surgical glove filled with distilled water. 50,51,52
Doppler imaging of the ophthalmic artery
The measurement of the velocity of blood flow in the ophthalmic artery through Doppler imaging has been tested in several studies aiming to determine the normal parameters of premature patients with or without ROP.53,54,55,56 It is a method that requires costly systemic ultrasound equipment but is available in many level-3 hospitals. It also requires specific technical knowledge and skills from the examiner for a proper identification of the ophthalmic artery in very young patients.
In our NICU at the Porto Alegre Clinics Hospital, we used LOGIC 5 (GE Healthcare, Little Chalfont, Buckinghamshire, UK) with a 7.5 MHz probe as a transducer in a study that analyzed 85 premature patients with BW ≤ and/or GA ≤ 32 weeks and performed weekly measurements from the first week after birth until discharge from the NICU for the longitudinal determination of blood flow values in the ophthalmic artery of VLBW preterm infants who did not develop ROP. We found the systolic velocity to vary significantly from 17.85 ± 5.30 cm/s on the first examination (1 week after birth) to 23.51 ± 5.63 cm/s at hospital discharge. Diastolic velocity also varied significantly, from 6.17 ± 1.13 cm/s to 6.94 ± 1.53 cm/s during the weeks following the preterm birth. With these values of normality, determined longitudinally in VLBW preterm infants, it would be necessary to establish values of blood flow velocity in the central artery of the retina in VLBW preterm infants who developed stage 3 of ROP, threshold ROP, or Type 1 prethreshold ROP, to know if Doppler imaging would be able to predict the need for further treatment for severe ROP in these patients. 53
Optical coherence tomography (OCT)
Optical coherence tomography (OCT) is an important investigational method recently introduced in ophthalmology. It offers much information, particularly about the macular and papillary regions. It allows the retina and macular region to be studied in cross sections from the vitreoretinal interface, internal limiting membrane and other retinal layers, Bruch's membrane complex/pigment epithelium, and choriocapillaris.
In patients with ROP, stage 2 or 3, OCT enables a very detailed examination of the anatomical conditions of the macular region, checking for the presence of vitreoretinal traction and intraretinal edema, and allowing a prognosis regarding the future vision ability of patients treated by laser.
Preserved macular and foveal anatomy has been reported in no more than 25% of patients affected by ROP, stage 2 or 3. In patients with ROP in later stages, ROP 4A or B, and in patients with ROP 5 who require surgical treatment, OCT shows important anatomical alterations and allows preoperative strategies to be defined, aiming to find the best surgical option on a case-by-case basis. OCT always allows a prognosis of visual recovery after the procedures. 57,58
DIFFERENTIAL DIAGNOSIS
The main congenital vitreoretinal diseases to be considered in the differential diagnosis of ROP are the Norrie disease of sex-linked recessive inheritance and the familial exudative vitreoretinopathy of autosomal dominant inheritance. Both are retinal development disorders and may show fundus aspects that are very similar to the later stages of ROP. The major clue pertaining to the differential diagnosis between ROP and these entities is that they usually occur in full-term children. 59
The main acquired disease to be considered in the differential diagnosis of cicatricial or regressive ROP is ocular toxocariasis with vitreoretinal disorganization, especially when the formation of vitreous lumens and peripheral granulomas occurs. ELISA test for toxicocariasis is used for this differential diagnosis.
ROP TREATMENT
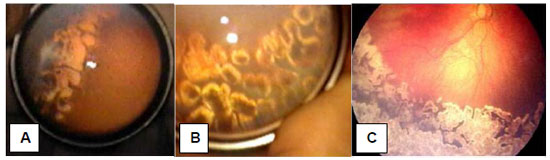
Cryotherapy of the peripheral avascular retina became the standard treatment for threshold ROP in 1988 based on the results of the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity, which confirmed that this treatment prevented ROP progression in a significant number of patients. 60 In recent times, cryotherapy has been set aside due to the selection of laser applied using BIO as the method of choice for the treatment of ROP. The results of the transpupillary photocoagulation treatment, with argon and diode lasers, are good, and the natural progression of ROP can be stopped in most patients treated with this technique. The treatment of ROP by transpupillary photocoagulation using laser has to be performed around 37 weeks post-conception (post-conception age = gestational age + weeks after birth), when ROP reaches the most dangerous stage of its progression (threshold ROP) or 36 weeks post-conception, when Type 1 prethreshold ROP occurs. Treatments performed after these post-conception ages usually have a worse prognosis for visual quality maintenance in the affected patient. 61
Laser photocoagulation can be performed in the surgical center under general anesthesia and in the NICU with sedation controlled by the neonatologist. Laser impact should be directed to the peripheral ischemic retina outside the demarcation ridge between the vascularized retina and the avascular retina, and it should be applied confluently, that is, laser impact marks should be very close to one another to ensure that all ischemic areas are covered by the laser beams (Figure 3). The intensity of the laser burn should be sufficient to produce a whitish injury on the avascular retina. If the plus disease persists for more than 14-21 days after the treatment, a second treatment with laser should be taken into account, which occurs in 10% to 15% of the cases and requires training and experience of the ophthalmologist in charge of this treatment in premature infants with important clinical instability.
In an attempt to improve the visual outcomes and using an approach that is more focused on the disease pathophysiology, many authors since 2009 have used anti-VEGF medication for the treatment of severe ROP. After many reports of cases were published with encouraging results obtained using bevacizumab (Avastin) for ROP treatment, the BEAT-ROP study in 2011 demonstrated a better efficacy of bevacizumab in multicenter prospective randomized studies for the treatment of ROP in stage 3 with plus disease in Zone I when compared to laser photocoagulation.62 Since then, many studies have been published demonstrating successful outcomes of both bevacizumab and ranibizumab in the treatment of severe ROP, both as an initial treatment of ROP and as a complementary treatment after initial photocoagulation in patients with severe ROP in Zone I as well as in patients with severe ROP in posterior Zone II. Of note, the ideal time to perform anti-VEGF intravitreal injection treatment should be at 35 or 36 weeks post-conception, when the largest amount of VEGF is released into the peripheral retina, that is, a little before the time considered ideal for laser treatment. Anti-VEGF treatments performed after 35 or 36 weeks post-conception can cause devastating consequences to the retina, especially if fibrotic tissue is already identified on fundus examination. 63,64,65,66
PROGNOSIS AFTER ROP TREATMENT
ROP usually regresses in most patients who develop only stage 1 or 2 because peripheral vascularization may be completed in the weeks following the preterm birth, leaving few residual anatomical and functional changes, which do not require any specific treatment. Such patients only need follow-up during the first years of life to prevent amblyopia and strabismus and to correct refraction errors, the incidence of which seems to be higher in preterm infants when compared to that in full-term infants. 67
When ROP reaches stage 3 and threshold disease or stage 2 or 3 and Type 1 prethreshold disease, the infant needs treatment because 50% of patients with threshold disease and more than 15% of patients with Type 1 prethreshold disease will present unfavorable anatomical and functional outcomes if no adequate treatment is provided. 68
The prognosis of treatment by diode laser photocoagulation in patients with threshold disease is good, and it is possible to avoid visual loss in about 70%-80% of the patients today.6970 However, the involvement of the macular region may result in a final visual acuity much below the expected result. Many of the treated children show later visual acuity that is not very satisfactory due to residual anatomical changes that may be located in the macular region or due to the occurrence of strabismus, amblyopia, or high refraction errors. 71
Eyes progressing to ROP 4A or B and ROP 5 stages requiring surgical treatment show much worse anatomical and functional prognosis even after retinopexy with scleral introflection or after vitrectomy through the pars plicata site, with or without lens preservation.
If preterm infants with a potential risk of developing severe ROP are identified early, they can receive more careful and personalized perinatal care focused on various risk factors. That would help planning the choice of the best time for the ophthalmologic treatment because the most debilitated children and those with worse postnatal prognosis are the ones at the greatest risk of ROP. More adequate perinatal management of premature patients at the risk of developing advanced ROP could lead to an efficient prevention of more severe forms of the disease, leading to a reduced incidence of childhood blindness.
REFERENCES
1. Gilbert C, Rahi J, Eckstein M, O'Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350(9070):12-4. http://dx.doi.org/10.1016/S0140-6736(97)01107-0
2. Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518-25. http://dx.doi.org/10.1542/peds.2004-1180
3. Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. Br J Ophthalmol. 2002;86(6):696-700. PMC1771164. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771164/#abstractid223760title
4. Terry T. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. I - Preliminary report. Am J Ophthalmol. 1942;25:203-4. http://dx.doi.org/10.1016/S0002-9394(42)92088-9
5. Terry T. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. II - Report of cases - clinical aspects. Am J Ophthalmol. 1943;29:36-53. PMC1315050. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1315050/pdf/taos00059-0283.pdf
6. Silverman WA. Retrolental fibroplasia: a modern parable. New York: Grune & Stratton; 1980. https://doi.org/10.1017/S0021932000013754
7. Heath P. Pathology of the retinopathy of prematurity, retrolental fibroplasia. Am J Ophthalmol. 1951 ;34:249. http://dx.doi.org/10.1016/0002-9394(51)91859-4
8. Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med J Aust. 1951 ;2(2):48-50. https://www.ncbi.nlm.nih.gov/pubmed/14874698
9. Crosse VM. Retrolental fibroplasia. Ulster Med J. 1952;21(1):32-5. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2479972/pdf/ulstermedj00162-0042.pdf
10. Cross KW. Cost of preventing retrolental fibroplasia? Lancet. 1973;2(7835):954-6. http://dx.doi.org/10.1016/S0140-6736(73)92610-X
11. Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3(1):26-32. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/10071898
12. Gilbert C. Retinopathy of prematurity--the "second lull"? Br J Ophthalmol. 2001;85(9):1017-9. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724130/pdf/v085p01017a.pdf
13. Gilbert C, Foster A. Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ. 2001;79(3):227-32. http://dx.doi.org/10.1590/S0042-96862001000300011
14. Moraes NSB, Bonomo PP, Almeida MFB. Retinopatia da prematuridade: estudo prospectivo de 1342 casos. Arq Bras Oftalmol. 1995;58(4 Supl):255
15. Bonotto LB, Moreira AT, Carvalho DS. Prevalence of retinopathy of prematurity in premature babies examined during the period 1992-1999, Joinville (SC): evaluation of associated risks-screening. Arq Bras Oftalmol. 2007;70(1):55-61. http://dx.doi.org/10.1590/S0004-27492007000100011
16. Schumann RF, Barbosa AD, Valete CO. Incidence and severity of retinopathy of prematurity and its association with morbidity and treatments instituted at Hospital Antonio Pedro from Universidade Federal Fluminense, between 2003 and 2005. Arq Bras Oftalmol. 2010;73(1):47-51. http://dx.doi.org/10.1590/S0004-27492010000100008
17. Pinheiro AM, Silva WA, Bessa CG, Cunha HM, Ferreira MA, Gomes AH. Incidence and risk factors of retinopathy of prematurity in University Hospital Onofre Lopes, Natal (RN)-Brazil. Arq Bras Oftalmol. 2009;72(4):451-6. http://dx.doi.org/10.1590/S0004-27492009000400005
18. Zin A, Florencio T, Fortes Filho JB, Nakanami CR, Gianini N, Graziano RM, et al. Brazilian guidelines proposal for screening and treatment of retinopathy of prematurity (ROP). Arq Bras Oftalmol. 2007;70(5):875-83. http://dx.doi.org/10.1590/S0004-27492007000500028
19. Lermann VL, Fortes Filho JB, Procianoy RS. The prevalence of retinopathy of prematurity in very low birth weight newborn infants. J Pediatr (Rio J). 2006;82(1):27-32. http://dx.doi.org/10.2223/JPED.1433
20. Fortes Filho JB, Barros CK, da Costa MC, Procianoy RS. Results of a program for the prevention of blindness caused by retinopathy of prematurity in southern Brazil. J Pediatr (Rio J). 2007;83(3):209-16. http://dx.doi.org/10.2223/JPED.1611
21. Fortes Filho JB, Eckert GU, Procianoy L, Barros CK, Procianoy RS. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye (Lond) 2009;23(1):25-30. http://dx.doi.org/10.1038/sj.eye.6702924
22. Fortes Filho JB. Retinopatia da prematuridade [artigo de revisão]. Rev Bras Oftalmol. 2006;65(4):246-58. http://hdl.handle.net/10183/71730
23. Graziano RM, Leone CR. Frequent ophthalmologic problems and visual development of extremely preterm newborn infants. J Pediatr (Rio J). 2005;81(1 Suppl):S95-100. http://dx.doi.org/10.1590/S0021-75572005000200012
24. Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940-85. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1310320/pdf/taos00033-0953.pdf
25. Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics. 1984;73(1):82-96. Abstract disponível em: http://pediatrics.aappublications.org/content/73/1/82
26. Fielder AR, Reynolds JD. Retinopathy of prematurity: clinical aspects. Semin Neonatol. 2001;6(6):461-75. http://dx.doi.org/10.1053/siny.2001.0091
27. Hellström A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001 ;8;98(10):5804-8. http://dx.doi.org/10.1073/pnas.101113998
28. Hellström A, Engstrom E, Hard AL, Bertsson-Wikland K, Carlsson B, Niklasson A, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112(5):1016-20. Abstract disponível em: http://pediatrics.aappublications.org/content/112/5/1016
29. Villegas BE, Fernandez MF, Gonzalez R, Gallardo Galera JM. Serum IGF-I levels in retinopathy of prematurity. New indications for ROP screening. Arch Soc Esp Oftalmol. 2005;80(4):233-8. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+15852164
30. Hellström A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, de Lacerda L, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87(7):3413-6. http://dx.doi.org/10.1210/jcem.87.7.8629
31. Engstrom E, Niklasson A, Wikland KA, Ewald U, Hellström A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57(4):605-10. http://dx.doi.org/10.1203/01.PDR.0000155950.67503.BC
32. Fielder AR, Shaw DE, Robinson J, Ng YK. Natural history of retinopathy of prematurity: a prospective study. Eye (Lond). 1992;6(Pt 3):233-42. http://dx.doi.org/10.1038/eye.1992.46
33. Fortes Filho JB, Eckert GU, Valiatti FB, Santos PGB, Costa MV, Procianoy RS. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP). Graefes Arch Clin Exp Ophthalmol. 2010;248:893-900. http://dx.doi.org/10.1007/s00417-009-1248-6
34. Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. a multivariate statistical analysis. Ophthalmologica. 2000;214(2):131-5. http://dx.doi.org/10.1159/000027482
35. Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18(2):130-4. http://dx.doi.org/10.1111/j.1365-3016.2003.00545.x
36. Procianoy RS, Garcia-Prats JA, Hittner HM, Adams JM, Rudolph AJ. An association between retinopathy of prematurity and intraventricular hemorrhage in very low birth weight infants. Acta Paediatr Scand. 1981;70(4):473-7. http://dx.doi.org/10.1111/j.1651-2227.1981.tb05725.x
37. Christiansen SP, Fray KJ, Spencer T. Ocular outcomes in low birth weight premature infants with intraventricular hemorrhage. J Pediatr Ophthalmol Strabismus. 2002;39(3):157-65. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+12051281
38. Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115(4):990-6. http://dx.doi.org/10.1542/peds.2004-1309
39. Fortes Filho JB, Costa MC, Eckert GU, Santos PG, Silveira RC, Procianoy RS. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011;158(3):372-6. http://dx.doi.org/10.1016/j.jpeds.2010.08.051
40. Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4(6):343-7. http://dx.doi.org/10.1067/mpa.2000.110342
41. Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):831-6. http://dx.doi.org/10.1007/s00417-008-1012-3
42. Hiraoka M, Shastry BS. Evaluation of the prothrombin gene polymorphism in patients with advanced retinopathy of prematurity. Genet Test. 2000;4(1):75-7. http://dx.doi.org/10.1089/109065700316525
43. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102(8):1130-4. http://dx.doi.org/10.1001/archopht.123.7.991
44. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. Arch Ophthalmol. 1987;105(7):906-12. http://dx.doi.org/10.1001/archopht.1987.01060070042025
45. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991 -9. http://dx.doi.org/10.1001/archopht.123.7.991
46. Hutchinson AK, Saunders RA, O'Neil JW, Lovering A, Wilson ME. Timing of initial screening examinations for retinopathy of prematurity. Arch Ophthalmol. 1998; 116(5):608-12. http://dx.doi.org/10.1001/archopht.116.5.608
47. Azad R, Chandra P, Khan MA, Darswal A. Role of intravenous fluorescein angiography in early detection and regression of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2008;45(1):36-9. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A++++18286961
48. Ng EY, Lanigan B, O'Keefe M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43(2):85-90. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+16598974
49. Maidana EJ, Matieli LC, Allemann N, Melo Jr LA, Morales M, Moraes NS. Ultrasonographic findings in eyes with retinal detachments secondary to retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2007;44(1):39-42. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+17274334
50. Tamburrelli C, Ricci B, Dicembrino M, Santo A. An ultrasonographic study of stage-5 retinopathy of prematurity. Ophthalmologica. 1998;212(6):381-8. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+9787227
51. Jokl DH, Silverman RH, Nemerofiky SL, Kane SA, Chiang MF, Lopez R, et al. Is there a role for high-frequency ultrasonography in clinical staging of retinopathy of prematurity? J Pediatr Ophthalmol Strabismus. 2006;43(1):31-5. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1618793/
52. Jokl DH, Silverman RH, Springer AD, Towers H, Kane S, Lopez R, et al. Comparison of ultrasonic and ophthalmoscopic evaluation of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2004;41(6):345-50. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2803061/?report=classic
53. ▲ Soares CR, Silveira RC, Procianoy RS. Ophthalmic artery blood flow in very-low-birth-weight preterm infants. Invest Ophthalmol Vis Sci. 2010;51(2):708-11. http://dx.doi.org/10.1167/iovs.09-4206
54. Neely D, Harris A, Hynes E, McNulty L, McCranor L, Siesky B, et al. Longitudinal assessment of plus disease in retinopathy of prematurity using color Doppler imaging. J AAPOS. 2009;13(5):509-11. http://dx.doi.org/10.1016/j.jaapos.2009.08.012
55. Baerts W, Wildervanck de Blécourt-Devilee M, Sauer PJ. Ambient light, ophthalmic artery blood flow velocities and retinopathy of prematurity. Acta Paediatr. 1993;82(9):719-22. http://dx.doi.org/10.1111/j.1651-2227.1993.tb1 2545.x
56. Romagnoli C, Papacci P, Zecca E, Giannantonio C, De Carolis MP, Tortorolo G. Normal neonatal values of ophthalmic and central retinal artery blood flow velocities. J Pediatr Ophthalmol Strabismus. 2001;38(4):213-7. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/?term=PMID%3A+11495308
57. Lago A, Matieli L, Gomes M, Baba NT, Farah ME, Belfort JR, et al. Stratus optical coherence tomography findings in patients with retinopathy of prematurity. Arq Bras Oftalmol. 2007;70(1):19-21. http://dx.doi.org/10.1590/S0004-27492007000100004
58. Joshi MM, Trese MT, Capone Jr A. Optical coherence tomography findings in stage 4A retinopathy of prematurity: a theory for visual variability. Ophthalmology. 2006;113(4):657-60. http://dx.doi.org/10.1016/j.ophtha.2006.01.007
59. Hutcheson KA, Paluru PC, Bernstein SL, Koh J, Rappaport EF, Leach RA, et al. Norrie disease gene sequence variants in an ethnically diverse population with retinopathy of prematurity. Mol Vis. 2005;11:501-8. Disponível em: http://www.molvis.org/molvis/v11/a58/
60. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988; 106(4):471-9. http://dx.doi.org/10.1001/archopht.1988.01060130517027
61. Fortes Filho JB, Eckert GU, Valiatti FB, Santos PGB, Costa MC, Procianoy RS. Postconceptional age at treatment of retinopathy of prematurity in inborn and referred preterm infants from the same institution. Arq Bras Oftalmol. 2011,74(4):251-4. http://dx.doi.org/10.1590/S0004-27492011000400004
62. Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603. http://dx.doi.org/10.1056/NEJMoa1007374
63. Yetik H, Gunay M, Sirop S, Salihoglu Z. Intravitreal bevacizumab monotherapy for type-1 prethreshold, threshold, and aggressive posterior retinopathy of prematurity-27 month follow-up results from Turkey. Graefes Arch Clin Exp Ophthalmol. 2015;253:1677. http://dx.doi.org/10.1007/s00417-014-2867-0
64. Nicoara SD, Nascutzy C, Cristian C, Irimescu I, Stefanut AC, Zaharie G, et al. Outcomes and prognostic factors of intravitreal bevacizumab monotherapy in zone i stage 3+ and aggressive posterior retinopathy of prematurity. J Ophthalmol. 2015; 2015:102-582. http://dx.doi.org/10.1155/2015/102582
65. Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015; 122:1008. http://dx.doi.org/10.1016/j.ophtha.2014.12.017.0
66. Arámbulo O, Dib G, Iturralde J, Duran F, Brito M, Fortes Filho JB. Intravitreal ranibizumab as a primary or a combined treatment for severe retinopathy of prematurity. Clin Ophthalmol. 2015;9:2027-32. http://dx.doi.org/10.2147/OPTH.S90979
67. Robinson R, O'Keefe M. Follow-up study on premature infants with and without retinopathy of prematurity. Br J Ophthalmol. 1993;77(2):91-4. http://dx.doi.org/10.1136/bjo.77.2.91
68. McNamara JA, Tasman W, Brown GC, Federman JL. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98(5):576-80. http://dx.doi.org/10.1016/S0161-6420(91)32247-4
69. Good WV. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233-50. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1280104/
70. Good WV. The early treatment for retinopathy of prematurity study: structural findings at age 2 years. Br J Ophthalmol. 2006;90(11):1378-82. http://dx.doi.org/10.1136/bjo.2006.098582
71. Holmstrom G, el Azazi M, Kugelberg U. Ophthalmological follow up of preterm infants: a population based, prospective study of visual acuity and strabismus. Br J Ophthalmol. 1999;83(2):143-50. http://dx.doi.org/10.1136/bjo.83.2.143


Funding source: None
Conflicts of interest: None
Received on:
November 14, 2016.
Accepted on:
November 24, 2016.