Alessandro José Rodrigues Daré; Leonardo Cunha Castro; Marina Rodrigues de Sunti
DOI: 10.17545/eOftalmo/2022.0005
ABSTRACT
Age-related macular degeneration is the principal cause of irreversible blindness in the developed world. There are some effective treatment options for neovascular age-related macular degeneration, with the current and preferred choice by ophthalmologists known as the ‘treat and extend’ (T&E) strategy. Injections are administered monthly until no sign of the disease activity, such as intraretinal fluid or subretinal, is observed on OCT, followed by a gradual extension of the treatment interval by two weeks. However, the maximum effective interval between injection applications is not yet established. This case report demonstrated the efficacy in a greater range than described in the literature; therefore, further investigations are necessary for the maximum effective interval determination.
Keywords: Mmacula and posterior pole degeneration; Active subretinal neovascular membrane; Choroidal neovascularization; Treat and extend strategy.
RESUMO
A degeneração macular relacionada à idade é a principal causa de cegueira irreversível no mundo desenvolvido. Existem algumas opções de tratamento eficazes para degeneração macular relacionada à idade neovascular, sendo a estratégia ‘tratar e estender’ a mais recente e a preferida pelos oftalmologistas, onde as injeções são realizadas mensalmente até que nenhum sinal de atividade da doença, como fluido intrarretiniano ou sub-retiniano, seja evidenciado na OCT, seguido por uma extensão gradual do intervalo de tratamento por 2 semanas. O que ainda não é consagrado é o máximo intervalo eficaz entre as aplicações; neste relato de caso demonstramos uma eficácia em um intervalo maior do que o descrito na literatura, sendo, portanto, necessária futuras investigações para essa determinação.
Palavras-chave: Degeneração da mácula e do polo posterior; Membrana neovascular subretiniana ativa; Neovascularização de coróide; Estratégia tratar e estender.
INTRODUCTION
Age-related macular degeneration (AMD) is the major cause of irreversible blindness in the developed world. Introduction the use of intravitreal antiangiogenic drugs has transformed the way wet (or neovascular) AMD is treated, enabling the prevention of visual acuity worsening that occurred in treatments with phototherapy (photocoagulation of the neovascular membrane, photodynamic therapy, among others), by preventing moderate visual loss in 90%-95% of patients and enabling visual improvement of 15 letters or more in 33% and 40% of the patients, respectively in the MARINA and ANCHOR pivotal studies. Notably, to reduce the inconvenience of monthly intravitreal injections, some treatment protocols have been developed to reduce treatment and patient visit frequencies without compromising the monthly treatment results. Among the treatment protocols developed, the Treat and Extend (T&E) strategy was extensively accepted by retinologists1 for reducing the number of treatment sessions and patient consultations required. Summarily, the Treat and Extend strategy consists of giving monthly intravitreal injections until no sign of the disease activity is observed, such as intraretinal or subretinal fluid shown on optical coherence tomography (OCT), accompanied by gradually extending the treatment for two weeks, since no recurrence of subretinal neovascular membrane activity was observed. However, the maximum safe interval between applications has not yet been established. Most studies mentioned 12 weeks as the maximum safe interval. In this case, we present a patient with wet AMD treated with intravitreal injections of the antiangiogenic drug while performing the Treat and Extend strategy up to an extended interval of 16 weeks, maintaining the efficacy and safety of the treatment.
CASE REPORT
A 77-year-old, white, retired female patient reported low visual acuity (LVA) in the left eye (OS) for three months. She had systemic arterial hypertension for ten years, treated with losartan potassium 50mg/day, and diabetes mellitus for ten years, in treatment with pioglitazone hydrochloride 30mg/day and metformin hydrochloride 1000mg/day. Additionally, implantation of phacoemulsification with an intraocular lens (IOL) was performed in both eyes six years ago and denied any trauma to the eye.
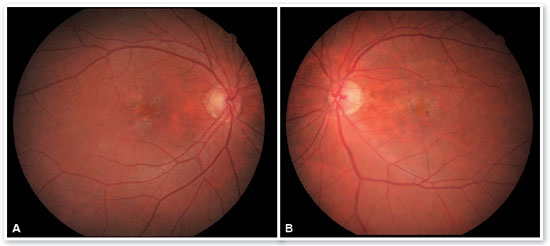
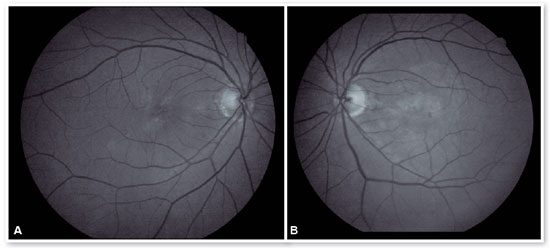
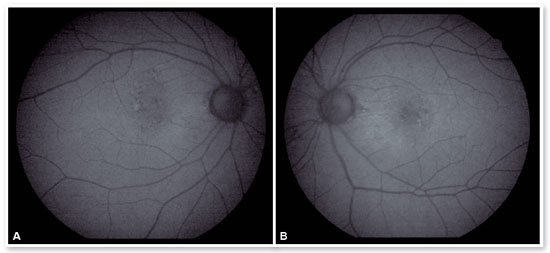
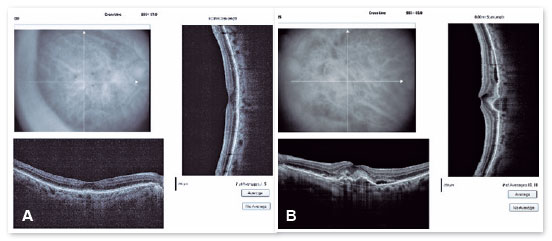
In the eye exam, she presented distance visual acuity (DVA) equal to 20/25 -1 in the right eye (OD) and 20/80+2 in the OS. Orthophoria was present in the far and near cover tests without correction. No changes in versions. Pupillary reflexes, direct and consensual, preserved. Anterior biomicroscopy showed a centered intraocular lens and posterior capsulotomy without other significant alterations. Intraocular pressure was 13 mmHg in both eyes. Fundoscopy in OU (Figures 1A and 1B): stained, well-defined optic nerve (ON) with 0.6 excavation, preserved caliber vessels, the macular region with hyperpigmentation, choroidal drusen, and rarefaction of the RPE (Retinal Pigment Epithelium) and periphery without alterations; in OS, she had epiretinal membrane (ERM) in the macular region and temporal parafoveal hypopigmentation with adjacent intraretinal hemorrhage, being the findings highlighted by RED FREE (Figures 2A and 2B). In autofluorescence (Figures 3A and 3B), hypoautofluorescent spots are observed interspersed with hyperautofluorescent spots in the macular region in OU. The OCT (Figures 4A and 4B) showed increased hyperreflective spots at the level of the RPE/Bruch’s membrane compatible with drusen in both eyes. ERM and subretinal hyperreflective material with adjacent subretinal fluid were evident in the left eye. Fluorescein angiography (Figure 5A - F) showed hyperfluorescent areas due to a window defect in the macular region in both eyes. In the left eye, there was late hyperfluorescence due to contrast extravasation in the macular region compatible with occult-type subretinal neovascular membrane.
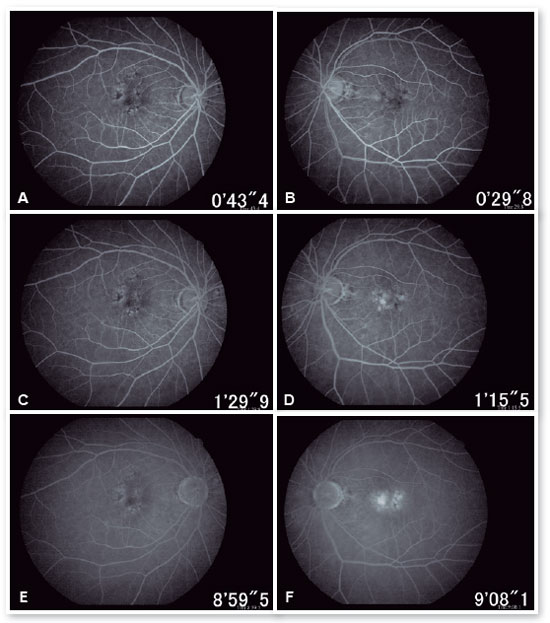
When doing multimodal analysis (Figures 6A - D), the diagnosis of dry AMD in the OD and exudative in the active OS was made, and treatment was started with intravitreal (IV) injections of the antiangiogenic drug (ranibizumab 10mg/ml) in the OS.
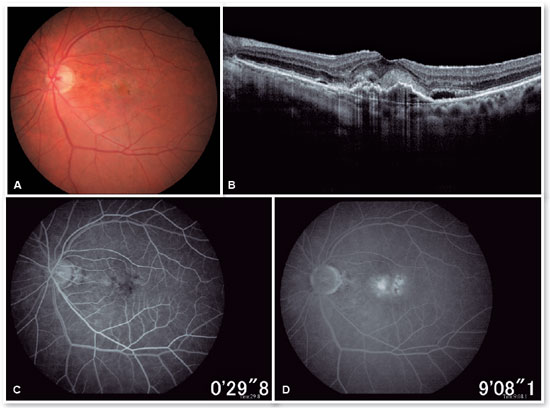
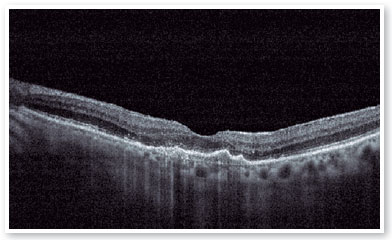
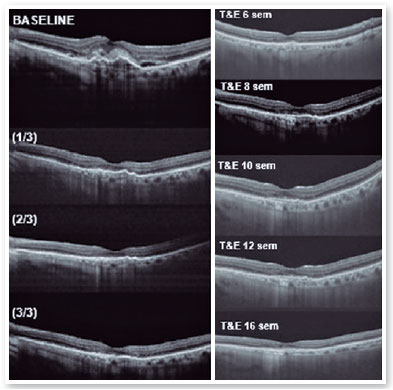
After performing the loading dose with three monthly IV injections, there was a significant improvement in the subretinal hyperreflective material and complete resolution of the subretinal fluid (Figure 7). T&E strategy was started with increments of 2 weeks between applications until reaching the 12-week interval, which was maintained for two consecutive visits without fluid recurrence. Therefore, we chose to increase the spacing to 14 weeks; however, for personal reasons, the patient returned at 17 weeks, maintaining no recurrence of the fluid and with significant anatomical and functional improvement (DVA of the OS: 20/40). It was then decided to maintain treatment with the maintenance of the 16-week treatment interval, with no recurrence of subretinal neovascular membrane activity during the follow-up period (19 months). In Figures 8 and 9, we can make a comparison from the baseline to the 16-week interval. The right eye remained stable throughout the patient’s follow-up period (Figure 10).
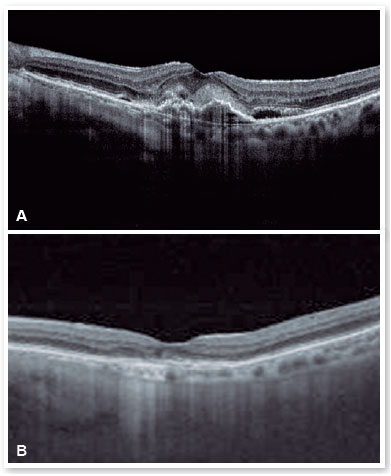
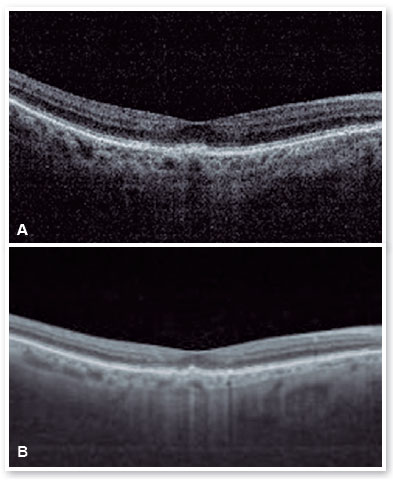
DISCUSSION
AMD is the leading cause of irreversible blindness in the developed world. Monthly or as-needed intravitreal antiangiogenic drug delivery strategies have been established as effective treatment options for neovascular AMD. More recently, the T&E regimen has been adopted in clinical practice. The decision for each injection and the treatment interval is based on the patient’s response until the last injection2,3. Both techniques result in comparable visual and anatomical gains, but fewer visits and intravitreal injections favor the T&E regimen 1,4.
Currently, T&E is the treatment strategy preferred by most retinal specialists 1. It represents a cost-effective and patient-centered option, providing individualized treatment and reducing the treatment burden by extending injection intervals when possible1,4.
Injections are given monthly until no sign of the disease activity, such as intraretinal or subretinal fluid, is evidenced on OCT, followed by a gradual extension of the treatment interval by two weeks to a maximum of 12 weeks5. The maximum interval between injections is not yet established. However, in the largest prospective T&E study, when writing this article, the two-week interval increase reached a maximum interval of 12 weeks 5.
The duration of VEGF-A (Vascular Endothelial Growth Factor) suppression differs between patients6. Therefore, adjusting the interval between applications based on each patient’s individual anatomical and functional results allows treatment, achieving optimal results while reducing the frequency of clinical visits beyond 12 weeks. The ALTAIR 7 results indicate that, with the T&E regimen, an interval of 12 weeks or more can be achieved in approximately 57-60% of patients, with 41-46% reaching the maximum interval of 16 weeks at the end of 2 years.
In the case presented, we kept the interval of 12 weeks, twice in a row, and as the condition remained stable without recurrence of retinal fluid or worsening of visual acuity, we chose to increase the interval to 14 weeks; however, the patient, for personal reasons, came only 17 weeks after the last IV injection, maintaining the anatomical and functional improvement (improvement in VA compared to baseline). For this reason, we maintained the spacing between injections to 16 weeks, with no recurrence of Subretinal Neovascular Membrane activity during the follow-up period (19 months).
So far, most studies have demonstrated efficacy and safety with an interval between injections of up to 12 weeks, establishing this as the maximum interval in clinical practice. However, efficacy was demonstrated in this case with a longer interval of 16 weeks; therefore, further studies are needed to determine the maximum effective interval.
REFERENCES
1. Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, Abdelfattah NS, Sadda SR, TREX-AMD Study Group. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration. TREX-AMD 1-year results. Ophthalmology. 2015;122(12):2514-22.
2. Spaide RF. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4): 679-80.
3. Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P, et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of Ranibizumab in exudative age-related macular degeneration. Retina. 2011;31(1):26-30.
4. Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, Clark WL, Abdelfattah NS, Sadda SR, TREX-AMD Study Group. Randomized Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: 2-Year Results of the TREX-AMD Study. Ophthalmol Retina. 2017;1(4):314-21.
5. Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A. Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol. 2014;98(9):1192-6.
6. Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol. 2016;100(11):1494-8.
7. Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, ALTAIR Investigators. Efficacy and Safety of Intravitreal Aflibercept Treatand- Extend Regimens in Exudative Age-Related Macular Degeneration: 52- and 96-Week Findings from ALTAIR, A Randomized Controlled Trial. Adv Ther. 2020;37(3):1173-87.
AUTHOR’S INFORMATION


Funding source: No specific financial support was available for this study
Conflict of interest: None of the authors have any potential conflict of interest to disclose
Received on:
June 24, 2021.
Accepted on:
June 1, 2022.