Antonio Ferreira de Mendonça Neto1; Marcela Alves Morais Vanazzi2; Gustavo Pascoal Azevedo1,2
DOI: 10.17545/eOftalmo/2020.0006
ABSTRACT
Cysticercosis is an endemic disease in humans, caused by foods contaminated with Taenia eggs. The parasite affects various organs, such as the heart, muscles, brain, and eyes. Eyes are the primary target in up to 46% of patients. Cysticercosis in the eye is characterized by remarkable inflammatory reaction, evolving with loss of visual acuity. Here we report the case of a 39-year-old patient with retrobulbar lesion secondary to neurocysticercosis who complained of low visual acuity in the right eye for 10 days. The examination showed retinal lesions; computed tomography of the skull showed the presence of calcified cysts highly suggestive of neurocysticercosis in the cerebral cortex; and magnetic resonance imaging of the orbits showed hypointense foci and contrast enhancement in the projection of the optic nerve head, suggesting retrobulbar cysticercosis.
Keywords: Cysticercosis; Neurocysticercosis; Retrobulbar neuritis.
RESUMO
Cisticercose é uma doença endêmica no homem ocasionada por alimentos contaminados de ovos de Taenia, o parasita acomete variados órgãos como coração, músculos, cérebro e olhos. Os olhos são o principal alvo em até 46% dos doentes, caracterizado por uma reação inflamatória importante, evoluindo com baixa de visão. Relataremos um caso de lesão retrobulbar secundária à Neurocisticercose (NCC). Paciente de 39 anos, queixando de baixa da acuidade visual no olho direito (OD) há 10 dias. O exame mostrou: lesões retinianas, tomografia computadorizada de crânio (TCC) com presença de cistos calcificados altamente sugestivos de neurocisticercose no córtex cerebral e ressonância nuclear magnética das órbitas (RNMO) com focos hipointensos e realce do contraste na projeção da cabeça do nervo óptico sugerindo cisticercose retrobulbar.
Palavras-chave: Cisticercose; Neurocisticercose; Neurite retrobulbar.
INTRODUCTION
Cysticercosis is a disease caused by Taenia solium larvae. Normally humans are definitive Taenia host; however, in some cases, humans become an intermediate host of the Cysticercus cellulosae larva. This is called human cysticercosis. This endemic disease is caused by water and food contaminated with eggs or even by self-infection1. A common disease in developing countries, cysticercosis is associated with several social aspects, such as individual health, group health, and public sanitation infrastructure2.
For the diagnosis of the disease in humans, it is important to describe the location of the cysticercus, which may be in the eye, the peripheral nervous system, or the central nervous system (CNS)1. Eye involvement occurs in approximately 13%-46% of cases3, and it can be either intra- or extra-ocular. Epidemiologically, children and young adults are the most affected groups.
The cysticercus releases a substance that produces an inflammatory reaction, visible as edema of the eyelids and orbital cellulitis. The lacrimal glands, orbital bones, and subperiosteum are little affected.Optic nerve impairment is usually evidenced as optic neuritis or disc intumescence, causing low visual acuity. This article presents a rare case suggestive of retrobulbar involvement secondary to neurocysticercosis (NCC).
CASE REPORT
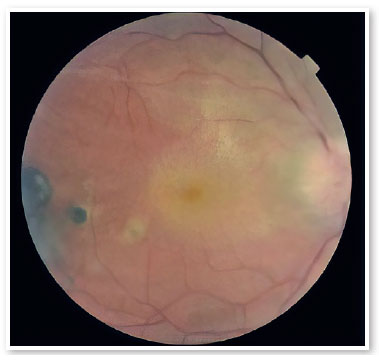
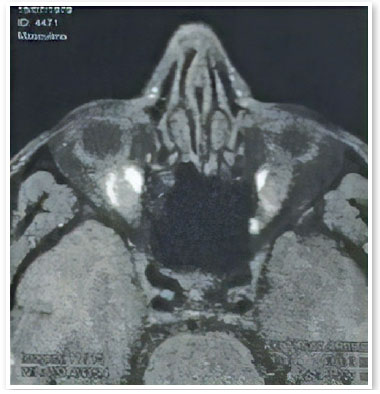
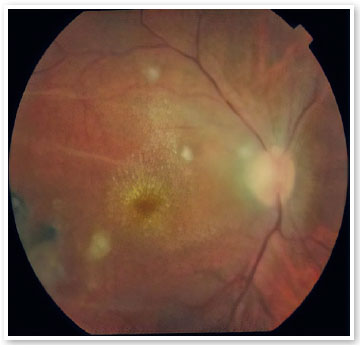
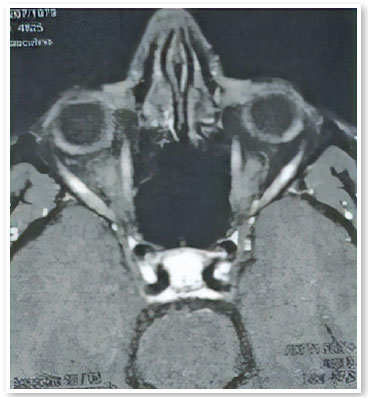
A male patient aged 39 years from the city of Porto Velho, State of Rondônia, Brazil, complained of low visual acuity in the right eye (OD) for 10 days. On ophthalmological examination, the best visual acuity (VA) was 20/400 and 20/20. Ocular motility, biomicroscopy, and applanation tonometry were within normal standards. OD fundoscopy showed papillary edema, peripapillary vasculitis, macular star, and temporal chorioretinitis lesions at various stages of evolution (Figure 1). At the time, a computed tomography (CT) scan of the skull showed calcified cysts highly suggestive of neurocysticercosis in the cerebral cortex. Nuclear magnetic resonance (NMR) of the orbits showed hypointense foci with contrast enhancement in the projection of the optic nerve head and along the extraconal fat - cyst - on the right side (Figure 2). Cerebrospinal fluid (CSF) cytology showed an increased leukocyte count. The enzyme-linked immunosorbent assay (ELISA) for cysticercosis was reagent in the serum and nonreagent in the CSF. ELISA for serum bartonellosis was nonreagent. Corticosteroid therapy with prednisone 1mg/kg/day was suggested. After 15 days of treatment, the patient presented VA improvement in OD to 20/200, reduction of papillary edema, improvement of the macular condition, and healing chorioretinitis lesions (Figures 3 and 4).
DISCUSSION
The involvement of cysticercus (from the Greek kustis, “bladder,” and kerkos, “tail”) in the human CNS was first described in 1588 by Rümler, who isolated the larva in the menynx of an epilepsy patient. In 1650, Panarolus found larvae in the corpus callosum of another patient with epilepsy. Laënnec proposed the designation of C. cellulosae owing to the predilection of cysticerci for human connective tissue. Only in 1853 did van Benedem describe the link between worms in the human intestine and parasitic cysticerci, a thesis corroborated by the works of Küchenmeister and Heubner in 1855 and Leuckart in 1856, whose articles permanently established the proposition that cysticercus is the cause of both swine and human cysticercosis.
The oldest articles on cysticercosis affecting eyes are from the 19th century, contemporary to the early use of fundoscopy devices. In 1829, Scholl and Soemmering reported a live Taenia solium larva in the anterior ocular chamber. In 1838, Baum described a cysticercus in the conjunctiva. In 1866, von Graefe reported the finding of a live larva in the vitreous humor for the first time. NCC in humans may be manifested with various signs, demonstrating a diversity of factors associated with age, immunity, and multiple other aspects.
In this reported case, CT and orbital NMR produced images highly suggestive of infection. However, some signs are nonspecific, and differential diagnosis with other CNS infections and neoplasms is still difficult. Currently, suggested NCC diagnostic criteria are based on clinical factors, images, serological markers, and epidemiological aspects. Thus, they are classified into four classes, based on the value of each diagnostic aspect1. Del Brutto’s criteria are presented below:
Absolute criteria
• Histopathological examination of the CNS showing cysticerci
• Cyst (scolex) visible in a CT or NMR scan of the skull
• Observation of subretinal larvae
Major criteria
• Strongly compatible lesions in a CT or NMR scan of the skull
• Cysticercus serological markers (electroimmunotransfer blot, EITB)
• Improvement of CNS cystic lesions after treatment
• Spontaneous improvement of minimal-unit lesions
Minor criteria
• Suggestive lesions in neuroimaging
• Compatible clinical signs of NCC
• Reagent ELISA for cysticercus in the serum or in the CSF
• Lesion outside the CNS
Epidemiological criteria
• Evidence of living with patients having cysticercosis in the family
• Subjects from endemic regions
• Regular visits to regions where the disease is common
Interpretation of Del Brutto’s criteria
• Definitive diagnosis:
⚬ One absolute criterion or two major criteria
⚬ One major criterion, two minor criteria, and one epidemiological criterion
• Probable diagnosis:
⚬ One major and two minor criteria, or one major, one minor, and one epidemiological criterion
⚬ Three minor criteria and one epidemiological criterion.
In the case reported, the patient presented one major criterion (calcified cysts suggestive of neurocysticercosis in the cerebral cortex), two minor criteria (reagent ELISA in the serum and a lesion outside the CNS). and one epidemiological criterion (coming from an endemic area), confirming the diagnosis of NCC.
For 25 years, the main form of therapy has been the use of cysticides, often combined with corticosteroids, seeking to reduce the amount and size of cysts and granulomas. However, no truly effective treatment is known for the control of NCC11.
When the larva is alive, it produces little inflammation; however, after the cysticercus is killed, the body’s immune response is very intense. The death of the larva while it is present in the eye often produces many toxic substances and leads to irreversible lesions in the eyeball12. The larva can be removed by laser, diathermy, or irradiation. A case has been reported in a patient with intravitreous cysticercus who underwent vitrectomy via pars plana and in the postoperative period, topical and oral corticosteroids were prescribed with gradual reduction, resulting in an improvement of the ocular inflammation and recovery of VA13.
In our study patient, an improvement of ocular inflammation was shown to occur with systemic corticotherapy, and surgical removal was not possible for histopathological study or cyst treatment. In this case, there was evidence of optic nerve involvement by the cysticercosis parasite, an unusual fact in the reviewed literature.
CONCLUSION
Ocular cysticercosis, although considered to have low prevalence, should be considered in the diagnostic hypotheses for intra- and extra-ocular diseases which present with myodesopsia and decreased VA, particularly in the presence of cysts or vesicles evidenced in the ophthalmological examination and associated with epidemiological criteria.
Therapy with antihelmintic cysticides can often be even more harmful, causing a significant release of toxic substances after the larva is killed. Owing to the frequent location of cysticerci in noble organs, treatment with systemic corticosteroids is often chosen to control local inflammation.
Therefore, it is necessary to perform new studies that investigate retrobulbar involvement by NCC because cysticercosis is endemic in some regions and can cause severe reduction of VA. Priority should be the prevention and control of parasites with public policies of basic sanitation and awareness of personal hygiene to avoid transmission and consequently, new cases of the disease.
REFERENCES
1. Pfuetzenreite MR, Ávila-Pires FD. Epidemiologia da teníase/ cisticercose por Taenia solium eTaenia saginata. Ciência Rural. 2000;30(3):541-8.
2. Guimarães RR, Orsini M, Guimarães RR, Catharino MAS, et al. Neurocisticercose: Atualização sobre uma antiga doença. Revista de Neurociência, 2010;18(4):593.
3. Duke-Elders S, Perkins ES. System of ophthalmology. Inflammations of the uveal tract: uveitis. Mosby.1966;3(9):478-87.
4. Santos R, Chavarria M, Aguirre AE. Failure of medical treatment in two cases of intraocular cysticercosis. Am J Ophthalmol, 1984; 97(2):249-50.
5. Malik SR, Gupta AK, Choudhry S. Ocular cysticercosis. Am J Ophthalmol, 1968;66(6):1168-71.
6. Demirci H; Singh AD. Essentials in Ophthalmology: Orbital inflammatory diseases and their differential diagnosis. Springer. 2015.
7. Brotto W. Aspectos neurológicos da cisticercose. Arq Neuro- Psiquiatr. 1947;5(3).
8. Garcia EA, Conde CK. Cisticercose intraocular bilateral: relato de um caso. Revista Acta Medica Misericordiae.1998;1(1):1-2.
9. Chavarría A, Roger B, Fragoso G, Tapia G, Fleury A, Dumas M, et al. TH2 profile in asymptomatic Taenia solium human neurocysticercosis. Microbes Infect. 2003;5(12):1109-15.
10. Del Brutto OH, Rajshekhar V, White Jr AC, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177-83.
11. García HH, Pretell EJ, Gilman RH, Martínez SM, Moulton LH, Del Brutto OH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249-58.
12. Reddy PS, Satyendran OM. Ocular cysticercosis. Am J Ophthal. 1964 Apr;57:664-6.
13. Pantaleão GR, Souza ADSB, Rodrigues EB, Coelho AI. Uso de corticóide sistêmico e intravítreo na inflamação secundária à cisticercose intra-ocular: relato de caso. Arq Bras Oftalmol. 2007; 70(6):1006-9.
AUTHOR’S INFORMATION



Financiamento: Declaram não haver.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Disclosure of potential conflicts of interest: Centro de Pesquisa em Medicina Tropical – CEPEM (CAAE: 19484419.4.0000.0011)
Received on:
December 10, 2019.
Accepted on:
June 17, 2020.