Isabella Wanderley de Queiroga Evangelista1; Camila Ribeiro Coutinho Honório2; Edivânia Pereira Leite3; Túlio Ivo Cordeiro Eulálio4
DOI: 10.17545/eoftalmo/2018.0014
ABSTRACT
OBJECTIVE: This study aimed to assess whether morphological features observed on spectral domain optical coherence tomography affect anatomical results in patients with neovascular age-related macular degeneration treated with ranibizumab.
METHODS: Before treatment, we assessed the presence of intraretinal fluid, subretinal fluid, an association between both, and retinal pigment epithelial detachment. After treatment, we assessed the association between these changes and the absence of retinal fluid. Twenty-two virgin eyes were included and underwent monthly injections of ranibizumab.
RESULTS: Before treatment, 22.7% of the eyes presented with intraretinal fluid only (group 1), 27.2% exhibited subretinal fluid only (group 2), and 45.4% had a combination of both (group 3). After treatment, 66.6%, 60.0%, and 33.3% of the eyes in groups 1, 2, and 3, respectively, presented no fluid, and none of the eyes with pigment epithelial detachment associated with the initial changes presented no fluid.
CONCLUSION: Eyes with retinal pigment epithelial detachment before treatment had the worst anatomical response to ranibizumab treatment. Eyes with a combination of both intraretinal and subretinal fluids had a worse response to treatment than those with intraretinal or subretinal fluid alone.
Keywords: Macular Degeneration; Ranibizumab; Tomography, Optical Coherence.
RESUMO
OBJETIVO: Este estudo objetivou verificar se características morfológicas à tomografia de coerência óptica de domínio espectral afetam os resultados anatômicos na degeneração macular relacionada à idade neovascular tratada com ranibizumabe.
MÉTODOS: Avaliou-se a presença de fluido intrarretiniano, de fluido sub-retiniano, de fluido intrarretiniano associado a fluido sub-retiniano, além de descolamento do epitélio pigmentar da retina, antes do tratamento, e a associação dessas alterações com a ausência de fluido retiniano, após o tratamento. Foram incluídos 22 olhos virgens de tratamento e submetidos a injeções mensais de ranibizumabe.
RESULTADOS: Inicialmente, 22,7% dos olhos apresentavam apenas fluido intrarretiniano (grupo 1), 27,2%, apenas fluido sub-retiniano (grupo 2) e 45,4%, combinação de fluidos intrarretiniano e sub-retiniano (grupo 3). Com o tratamento, os percentuais de olhos que se apresentaram sem fluido foram 66,6, 60,0 e 33,3% para os grupos 1, 2 e 3, respectivamente. Nenhum dos olhos com descolamento do epitélio pigmentar associado às alterações iniciais se apresentou sem fluido após o tratamento.
CONCLUSÃO: Olhos que apresentavam descolamento do epitélio pigmentar inicialmente exibiram a pior resposta anatômica ao tratamento com ranibizumabe. Olhos com a associação de fluidos intrarretiniano e sub-retiniano responderam pior ao tratamento do que aqueles com fluido sub-retiniano ou fluido intrarretiniano como alteração isolada.
Palavras-chave: Degeneração Macular; Ranibizumab; Tomografia de Coerência Óptica.
RESUMEN
OBJETIVO: Este estudio tuvo como objetivo verificar si las características morfológicas a la tomografía de coherencia óptica de dominio espectral afectan los resultados anatómicos en la degeneración macular relacionada a la edad neovascular tratada con ranibizumab.
MÉTODOS: se ha evaluado la presencia de líquido intrarretiniano, de líquido subretiniano, de líquido intrarretiniano asociado a líquido subretiniano, además de desgarro del epitelio pigmentario retiniano, antes del tratamiento, y la asociación de esas alteraciones con la ausencia de líquido retiniano tras el tratamiento. Fueron incluidos 22 ojos vírgenes de tratamiento y sometidos a inyecciones mensuales de ranibizumab.
RESULTADOS: Inicialmente, el 22,7% de los ojos presentaban solo líquido intrarretiniano (grupo 1), 27,2%, solo líquido subretiniano (grupo 2) y 45,4%, combinación de líquido intrarretiniano y subretiniano (grupo 3). Con el tratamiento, los porcentajes de ojos que se presentaron sin líquido fueron 66,6, 60,0 y 33,3% para los grupos 1, 2 3, respectivamente. Ninguno de los ojos con desgarro del epitelio pigmentario asociado a las alteraciones iniciales se presentó sin fluido tras el tratamiento.
CONCLUSIÓN: Los ojos que presentaban desgarro del epitelio pigmentar inicialmente mostraron la peor respuesta anatómica al tratamiento con ranibizumab. Ojos con la asociación de fluidos intrarretiniano y subretiniano respondieron de peor manera al tratamiento que aquellos con líquido subretiniano o líquido intrarretiniano como alteración separada.
Palabras-clave: Degeneración Macular; Ranibizumab; Tomografía de Coherencia Óptica.
INTRODUCTION
Antiangiogenic therapy has revolutionized the treatment of neovascular age-related macular degeneration (nAMD), which is the leading cause of permanent vision loss among older adults. In addition, modern diagnostic and monitoring methods, such as spectral domain optical coherence tomography (SD-OCT), have enabled a better understanding of the disease and of the structural damage that it causes to the macula, thus contributing to a significant decrease in the number of cases of blindness and improvement in the quality of life of patients.
Despite all this progress, currently, the most widely used classification of exudative AMD is the one that was proposed 20 years ago by the Macula Photocoagulation Study Group1, which was based on fluorescein angiography findings. It was used to determine the patients who would benefit from thermal laser photocoagulation to eradicate the entire neovascular complex and was aimed at the precise demarcation of the lesion by determining whether its location was extrafoveal, juxtafoveal, or subfoveal and, according to the fluorescence pattern, whether it was “classic” or “occult.” Extremely useful in the pre-antiangiogenic era, it failed to show any difference in the therapeutic response between these two types of lesions when it was used in the large clinical trials ANCHOR and MARINA, which dramatically changed the expectations for nAMD2,3.
However, as clinical professionals, we frequently observe considerable variations in individual patient’s responses to antiangiogenic treatment, which creates an interest to explore and understand the factors involved in these results.
The objective of this study is to assess whether morphological features observed on SD-OCT affect the anatomical results in eyes with nAMD treated with ranibizumab (Lucentis®, Novartis Pharma Stein AG, Stein, Swiss).
METHODS
A descriptive clinical study of the case-series type, in which features of macular morphology were assessed by SD-OCT before and after treatment with ranibizumab (Lucentis®). The study was approved by the local ethics committee before its beginning, and all procedures were performed in accordance with the Declaration of Helsinki. The sample included patients diagnosed with nAMD who had never received treatment before. Patients who had had prior treatment for AMD or those who also had other retinal diseases were excluded, as were those who had the first treatment cycle interrupted for any reason (such as non-attendance or lack of access to medicine).
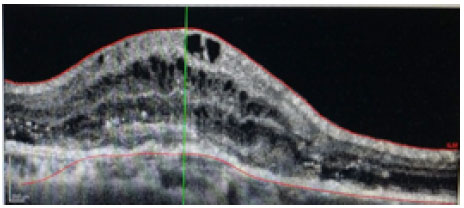
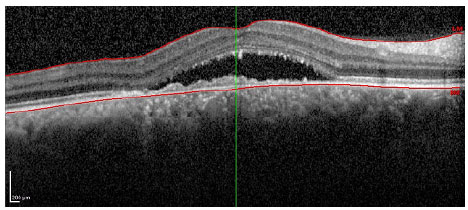
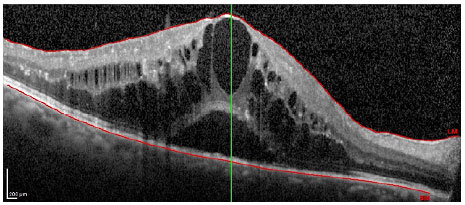
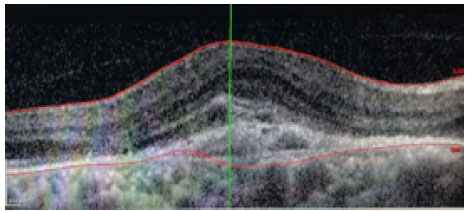
Patients received monthly injections of ranibizumab (Lucentis®) until the best corrected visual acuity was stable for three consecutive measurements during the treatment. The equipment used for the morphological study of the macula was the Spectralis® platform (Heidelberg Engineering, Heidelberg, Germany). The parameters assessed before treatment were the presence of intraretinal fluid (IRF; figure 1), subretinal fluid (SRF; Figure 2), both IRF and SRF (Figure 3), and retinal pigment epithelial detachment (DEP; figure 4), and after the treatment, the association of these changes with the absence of retinal fluid was assessed.
RESULTS
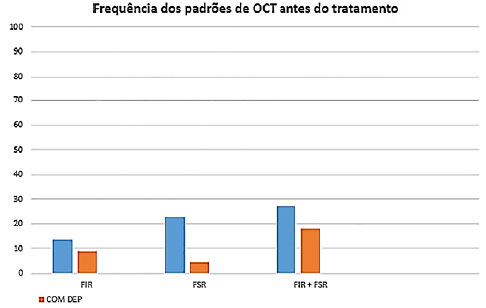
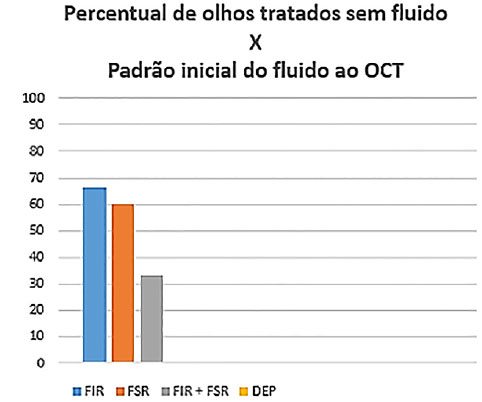
The study included 22 eyes from 21 patients, with a mean age was 70.3 years and mean number of injections of 4.5. Of the total number of eyes, 22.7% presented IRF only (group 1), 27.2% exhibited SRF only (group 2), and 45.4% had a combination of both (group 3) (figure 5). After treatment, the percentage of eyes presenting no fluid was 66.6%, 60.0%, and 33.3% for groups 1, 2, and 3, respectively. None of the eyes with pigment epithelial detachment associated with the initial changes presented no fluid after treatment, regardless of their initial fluid pattern (IRF, SRF, or both IRF and SRF) (figure 6).
DISCUSSION
Anti-VEGF therapies have been shown to reduce or reverse vision loss in patients with nAMD. However, because of the variability of responses among different patients and fluctuations in the responses of a single patient, predictors of anti-VEGF treatment results have been investigated4,5.
Previous studies have revealed the association of genetic, demographic, and clinical characteristics with the response to antiangiogenic therapy for nAMD6,7,8. OCT morphological features have also been proposed as the basis for a new classification of the disease9. The many advantages of using SD-OCT to improve the accuracy of the prognosis of age-related macular degeneration are evident—it is a fast, non-invasive method that is already part of the workup for this disease.
Our study investigated whether baseline OCT features enable the differentiation of anatomical responses at the first treatment cycle with ranibizumab (Lucentis®). In the study sample, the initial presence of DEP was associated with the persistence of retinal fluid in all cases. The combined presence of IRF and SRF at baseline also correlated with worse responses to treatment when compared with the presence of only one fluid pattern as an isolated change.
There are some limitations related to our study design. It was a small, non-randomized and retrospective study with a limited follow-up period. In addition, the study did not include genetic and demographic variables and did not investigate the association between morphological features and functional results. However, it is one of the few studies in the literature involving the macular structure and therapeutic response in patients with nAMD.
Eyes with exudative age-related macular degeneration presenting DEP initially showed the worst anatomical response to treatment with ranibizumab (Lucentis®). Eyes with both IRF and SRF had a worse response to treatment with this drug than those with only IRF or SRF as an isolated change.
REFERENCES
1. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1991;109(8):1109-14.
2.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-44.
3.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-31.
4.Ying GS, Maguire MG, Daniel E, Ferris FL, Jaffe GJ, Grunwald JE, et al.; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. Association of Baseline Characteristics and Early Vision Response with 2-Year Vision Outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2015;122(12):2523-31.e1.
5.Ying GS, Huang J, Maguire MG, Jaffe GJ, Grunwald JE, Toth C, et al.; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-9.
6.Krishnan R, Arora R, De Salvo G, Stinghe A, Severn PS, Pal B, et al. Vitreomacular traction affects anti-vascular endothelial growth factor treatment outcomes for exudative age-related macular degeneration. Retina. 2015;35(9):1750-6.
7.Brantley MA Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114(12):2168-73.
8.Smailhodzic D, Muether PS, Chen J, Kwestro A, Zhang AY, Omar A, et al. Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2012;119(11):2304-11.
9.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30(9):1333-49.



Funding: No specific financial support was available for this study
CEP Approval: CAAE: 50979415.6.0000.5183 (Hospital Universitário Lauro Wanderley/UFPB)
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclos
Received on:
May 15, 2018.
Accepted on:
May 25, 2018.