Daniel Amorim Leite1; Daniel Vítor Vasconcelos Santos2; Fábio Medina Rodrigues Rocha3; Marco Antônio Guarino Tanure4
DOI: 10.17545/e-oftalmo.cbo/2015.36
ABSTRACT
OBJECTIVE: This study aimed to present a broad overview of endothelial transplants and outline the techniques currently used and the advantages, disadvantages, and main challenges of advancements in the procedure.
METHODS: A descriptive study on the most frequently used techniques for endothelial transplantation based on the main articles published on the topic.
RESULTS: There have been considerable advancements in endothelial transplants in recent decades. Thus, the number of transplants performed has increased. Despite these advances and the advantages of endothelial transplantation, the assessment of the visual quality of the grafts is yet a challenge.
RESULTS: Analysis of the optical interface of endothelial transplants can be used to understand variations in visual quality experienced by patients who underwent different corneal graft preparation techniques. This analysis may aid in the understanding of unsatisfactory visual results after endothelial transplants, which may occur despite excellent anatomical results.
Keywords: Corneal Transplantation, Descemet Stripping, Endothelial Keratoplasty Endothelium, Corneal
RESUMO
OBJETIVO: O objetivo deste estudo foi apresentar uma visão abrangente dos transplantes endoteliais, explicando as técnicas realizadas atualmente, vantagens, desvantagens e os principais desafios de sua evolução.
MÉTODOS: Estudo descritivo das principais técnicas de transplante endotelial realizadas, com base nos principais artigos publicados sobre o tema.
RESULTADOS: Existe uma considerável evolução dos transplantes endoteliais nas últimas décadas. Dessa forma, o número de transplantes realizados apresenta um crescente. Apesar dos avanços e vantagens dos endoteliais, a avaliação da qualidade visual dos enxertos preparados ainda é um desafio.
CONCLUSÃO: A análise da interface óptica dos transplantes endoteliais é um dos caminhos para o entendimento da variação da qualidade visual de pacientes submetidos a diferentes técnicas de preparo dos enxertos corneanos. Esta análise poderá ajudar a compreender resultados visuais insatisfatórios com os transplantes endoteliais, apesar do excelente resultado anatômico.
Palavras-chave: Transplante de Córnea, Ceratoplastia Endotelial com Remoção da Lâmina, Limitante Posterior, Epitélio posterior
INTRODUÇÃO
The cornea is composed of five layers: the epithelium, Bowman's layer, the stroma, Descemet's membrane, and the endothelium.1 In 2013, Dua proposed the presence of a pre-Descemet layer termed Dua's layer. This observation was the result of a study regarding patients who had undergone anterior lamellar transplants using the big bubble technique.2
The endothelium acts as a pump that facilitates fluid regulation and the consequent transparency of the cornea. Endothelial loss may occur because of aging, hereditary diseases, or surgical trauma. This loss may cause endothelial dysfunction with subsequent corneal edema.3 The human endothelium does not have the capacity to regenerate in vivo, a young adult possesses 3500 cells/mm2 and a mean central corneal pressure of 550 µm.1
Fuchs's endothelial dystrophy was the main reason for penetrating keratoplasty in the US in 2011 (20.8%). It was the most common indication for endothelial transplant (48%).5 Pseudophakic bullous keratopathy is another indication for endothelial transplantation. In the year 2000, 22.3% of transplants in the US were performed because of pseudophakic bullous keratopathy. However, this number has been decreasing with the improvement of phacoemulsification techniques.6 Pseudophakic bullous keratopathy is yet the second most common reason for the indication of endothelial transplantation in the US; it is responsible for 19.2% of transplants.5 The development of bullous keratopathy is often associated with the subclinical presence of Fuchs's endothelial dystrophy. Cases may occur early or late in the postoperative period.
Other reasons for endothelial transplantation account for 12.3% of cases in the US. They include post-transplant penetrating failure, iridocorneal endothelial syndrome, and congenital hereditary endothelial dystrophy. Endothelial retransplantation occurred in 8.5% of cases. Less commonly, transplantation can be indicated for pain management or cosmetic appearance in painful cases of bullous keratopathy with no visual prognosis.5
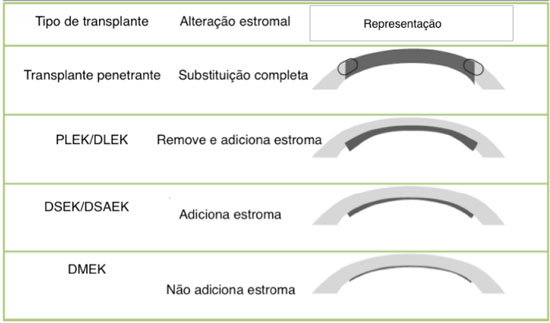
The idea of selectively replacing damaged layers of the cornea has been credited to von Hippel in 1886. The first successful cornea transplant was not performed until 1905 by Zirm.7 For decades, lamellar transplants were disregarded in favor of penetrating transplants. In 1956, Tillett performed a procedure characteristic of a posterior lamellar transplant, including a deep lamellar dissection of the recipient's cornea and sutures to attach the donor graft. Therefore, the procedure was highly complex and resulted in a high rate of complications, such as angle-closure glaucoma because of synechiae and endothelial failure.8 In 1998, Melles proposed the use of an air bubble rather than sutures to attach the graft. This began the modern era of endothelial surgery, which is referred to as posterior lamellar keratoplasty. However, dissecting the donor and recipient cornea is yet a challenge. Terry modified the technique with the introduction of new instruments and the use of an artificial chamber composed of cohesive viscoelastic material, a procedure that he termed deep lamellar endothelial keratoplasty.5 However, the techniques were not popularized because of their complexity and the limited visual acuity obtained because of the interface generated by two manually dissected surfaces.9 In 2004, Melles reported of a technique involving the stripping of Descemet's membrane, which avoided the manual deep stromal dissection procedure and generated a smoother surface for maintaining contact with the donor cornea. This process was termed Descemet's stripping endothelial keratoplasty (DSEK). In 2006, Gorovoy proposed the use of a microkeratome, which enabled easier the donor graft preparation. The procedure was termed Descemet's stripping automated endothelial keratoplasty (DSAEK).3 In 2006, Melles experimentally presented the Descemet's membrane endothelial keratoplasty (DMEK) technique. In this procedure, only the endothelium and Descemet's membrane were transplanted, with a theoretically smoother surface than the one with the presence of residual stroma, as in DSEK and DSAEK.9 Tappin showed that this technique may be performed in clinical practice using a cannula to implant the graft, a process termed true endothelial cell (Tencell) transplantation (Figure 4).10
2. METHODOLOGY
Descriptive analysis of the techniques used for endothelial transplantation, highlighting peculiarities and the visual results obtained.
3. RESULTS
The number of endothelial transplants has increased over the years with the transition from penetrating to lamellar transplantation. In 2005, only 1,429 endothelial transplants were performed. This number increased to 18,221 in 2009, when endothelial transplants represented 30.5% of the transplants performed in the US. This number tripled between 2007 and 2008 alone.3 Penetrating transplants decreased from 45,821 in 2005 to 23,269 in 2009.6 In 2011, the number of endothelial transplants was greater than the number of penetrating transplants in the US.5 Approximately 25,000 surgeries are performed per year because of endothelial diseases and correspond to 57% of transplant indications.3 In Brazil, a total of 14,696 corneal transplants were performed in 2011.11 There is no data regarding the number of lamellar transplants performed.
There are currently three main techniques available for endothelial transplantation: DSEK, DSAEK, and DMEK. The first two techniques differ in terms of their modes of preparation; however, the insertion, positioning, and attachment techniques are similar. The third technique offers its own preparation, insertion, positioning, and attachment techniques. DSEK is not used because of the difficulty of execution; however, the cost of the operation is lower than that of DSAEK because of the use of spatulas rather than a microkeratome. The donor cornea is attached to a synthetic chamber; therefore, it requires a corneoscleral rim of at least 2 mm in size. The deep stromal dissection is performed with spatulas. The dissection tends to be as deep as possible. With regard to DSAEK, a microkeratome is used to create the donor graft, which rendering the procedure easier. The cornea can be dissected using a microkeratome-assisted double-pass technique to produce thinner grafts, as proposed by Busin. Using ultrasonic pachymetry before creating the donor graft may aid in the choice of which microkeratome head thickness to use. In addition, the previous use of optical coherence tomography may aid in the microkeratome head choice.
The DMEK technique, originally described by Melles, is essentially manual, which extends the learning curve. It is performed by stripping Descemet's membrane 360° at the level of Schwalbe's line.19 To locate the membrane, trabecular pigmentation can be used as a reference.20 This maneuver releases the area of greatest adhesion from the Descemet/endothelial complex. The important part of this step is to guarantee that there are no areas of the complex attached to Schwalbe's line to minimize risks of tearing. Trypan blue solution is used to improve visualization of the graft. Next, the complex is dissected toward the cornea with the help of forceps. This initial dissection is performed up to 4 mm from the center of the cornea; this prevents the need to cover the entire 360°. Partial peripheral corneal trephination of the central area, approximately 8 mm in diameter, is performed (size varies based on the case). This prevents the need for a total peripheral corneal trephination and guarantees complete separation of the centrally and peripherally trephined area. This is important for preventing tearing during graft preparation. Descemet's stripping is then complete; the donor cornea is covered with a balanced salt solution (BSS), which reduces surface tension and avoids graft tearing.20 This technique of covering the donor graft is termed the submerged cornea using backgrounds away or SCUBA technique.22 The donor graft is then formed; because of its elastic properties, it tends to remain in a scroll-like shape, with the endothelial face rolled inside.
Endothelial transplant techniques have enabled the use of non-viable corneas in penetrating transplants. These corneas include those with anterior leukomas, corneas that have undergone refractive surgery, and infants' and children's corneas.(4) Armou et al. reported similar rates of endothelial density between corneas with and without alterations to the anterior portion. There has recently been an increase in the demand for transplant tissues. This increase has been accompanied by a growing number of potential cornea donors who have undergone or will undergo refractive surgery. These patients' corneas may be harvested for endothelial transplantation. One concern over the use of these corneas is that the alterations in the anterior portion may be transmitted to the recipient graft during the dissection and topographic alterations in patients who have undergone refractive surgery. Although these situations do occur, they appear to have limited influence over visual acuity.
Extreme cases have benefited from these techniques: eyes on which vitrectomies have been performed, cases of aniridia, or eyes on which several glaucoma surgeries have been performed. In these situations, there is a greater risk of graft detachment and greater endothelial loss.26 A technique to aid in these situations is using Prolene sutures to attach the donor graft. There are reports of success is eyes with developmental anomalies in the anterior segment, such as congenital hereditary endothelial dystrophy (CHED), microcornea, buphthalmia, and Peters plus syndrome. Because of good results, this surgery has increasingly been indicated earlier to avoid ambliopia and corneal opacity. In cases of Peters plus syndrome, there is greater difficulty because of iridocorneal synechiae, which, in turn, hinders Descemet's stripping and donor graft adhesion.31 In addition, patients who undergo penetrating transplantation and who exhibit endothelial failure may undergo endothelial transplantation. Currently, endothelial failure is one of the main indications for penetrating retransplantation; it represents a total of 18% to 40% of the indications. Rates of graft detachment and endothelial loss are higher in these cases than in eyes in which previous transplants have not been performed.32 In these cases, Descemet's membrane stripping in the recipient may not be performed because graft dehiscence may occur during the maneuver. There is a report of the use of endothelial grafts in cases of possible corneal perforations (descemetocele), which provides tectonic support until the fragile area is completely recovered.34
Dirisamer described some cases of endothelial graft detachment; however, the cornea recovered its transparency. This detachment occurred within a few hours after the transplant, which raises the question of whether the limited apposition time between the structures would be enough to promote endothelial cell repopulation or whether this would occur through a possible stimulation for the multiplication of endothelial cells. These cases comprise Descemet membrane endothelial transfer (DMET).35 Three situations may be listed in cases of corneal clearance without appropriate graft apposition. The first situation is one in which descemetorhexis has been incomplete; in this case, this residual portion has been capable of causing corneal clearance. The second and third possibilities involve the migration of donor graft cells toward the recipient stroma and the stimulation of endothelial stem cells.35 Another option is to divide of the cases into two groups: those with endothelial cell dysfunction, the main representation of which is Fuchs's endothelial dystrophy, and those with permanent lesions, the main representation of which is pseudophakic bullous keratopathy. In the first group, this endothelial dysfunction may be compensated by the removal of the damaged tissue, which would stimulate residual endothelium recovery. There are reports that descemetorhexis alone, without the insertion of donor endothelium, would be enough of a stimulus for endothelial recovery. Meanwhile, in the permanent endothelial lesion group, corneal recovery requires an endothelial transplant. The main disadvantage of this technique is the unpredictability of endothelial recovery and the low endothelial cell count obtained.35
Complications such as pupillary block (prevented by using an iridectomy), graft decentration or detachment, and rejection may occur in cases of endothelial transplantation.16 Detachment rates vary widely (from 1% to 82%), but the average is below 3%.5 With regard to DMEK, these rates vary widely, similar to rebubbling rates; slight graft detachments were previously treated differently: some surgeons choose to treat any detachment, whereas others only monitor certain cases. Rates of rejection in cases of DSAEK/DSEK vary among studies, from 0% to 46%. However, the average rate is 10%.5 Anshu et al. performed a comparative study on rejection rates among the DMEK, DSEK/DSAEK, and penetrating transplant techniques. The incidence of rejection was 0.7%, 9%, and 17%, respectively. Thus, the chance of rejection after the DMEK technique 15 times lower than that after the DESK technique and 15 times lower than that after the penetrating transplant.20 Primary graft failure is another possible complication. In penetrating transplants, the chance of rejection varies from <1% to 3%. In DSEK/DSAEK cases, the chance of rejection varies from 0% to 29%, a result that suggests that surgical trauma may be one of the main causes of failure. This rate tends to decrease as surgeons gain experience. Price described a primary failure rate of 8% in cases of DMEK in an initial study on 60 eyes, whereas Dapena reported 11 failures (9.2%) in a series of 120 eyes.8 Endothelial loss in the first few months tends to be greater when the endothelial transplant techniques are used, but its rate is eventually equal to that of penetrating transplantation. Average endothelial losses in DSEK and DMEK techniques are similar at 6 months and 1 year (endothelial loss of 36%).20 Finally, secondary glaucoma may occur at rates that vary from 0% to 54%. Etiology includes angle-closure, inflammation, and corticosteroid-induced glaucoma.13
DISCUSSION
Recent advances in corneal surgery techniques have resulted in an increase in the number of lamellar keratoplasty cases relative to cases of penetrating transplants. The selective substitution of only the compromised layer of the cornea maintains the integrity of the eyeball, both during and after the surgical procedure. Diseases affecting the anterior layers of the cornea may be treated using deep lamellar transplant techniques in which the entire stroma is removed to keep Descemet's membrane and the endothelium healthy. In cases of endothelial diseases, only this layer is selectively replaced.37
The advantages of endothelial transplants include surgical procedures performed on a closed eye, faster visual recovery, reduced induction of astigmatism, a lower incidence of suture-related complications, the preservation of the corneal structure, and a lower incidence of rejection because of the lower antigenic load of the corneal tissue. Another possible advantage is using the same cornea for two recipients, one of whom will undergo deep anterior lamellar keratoplasty (DALK) and the other who will undergo DMEK. Disadvantages include a more extensive learning curve, more financial investment than that involved in penetrating transplants (particularly with regard to DSAEK), and some unsatisfactory visual results, even with the anatomical success of the transplant.1
When only the DMEK technique is used, there may be other advantages in addition to the previously cited examples. They include fewer high-order aberrations, lower costs, and the substitution of only the corneal layer of interest, without the introduction of other components (such as the stroma). This renders the procedure optically neutral. The disadvantages of this specific type of technique include the limited number of trained surgeons, patient positioning in the post-operative period, and the frequent need for rebubbling, which requires more intense postoperative care.10 Most patients who undergo DSEK/DSAEK reach a visual acuity of 0.5, but only 10% to 25% reach ≥0.8. Conversely, 75% of patients who undergo DMEK reach a visual acuity of ≥0.8 within the first 6 months after surgery.38
However, the greatest challenge of DSEK/DSAEK is to understand why transplants with excellent anatomical success may have unsatisfactory functional results. It is difficult to distinguish among the influences of confounding variables; they may be associated with this result. These variables include prior ocular conditions (retinal disease, glaucoma, and opacity). Various factors may affect visual quality in eyes on which keratoplasty is performed. Factors involving the ocular surface and the anterior segment, including the lacrimal film complex, low-order corneal aberrations, anterior or posterior opacity, and pupillary decentrations may be responsible for unsatisfactory visual results.40 Dirisamer et al. evaluated the possible causes of low visual acuity in patients who had undergone DMEK after unsatisfactory visual acuity was achieved using DSEK. Explanations include late-onset graft failure and detachment, and the latter contributes to stromal edema and increased reflectivity in the stromal interface in DSEK/DSAEK. This is observed both biomicroscopically and when confocal microscopy is used. Normal reflectivity is observed using DMEK, whereas other techniques revealed a certain degree of opacity in 75% of cases. Another variable observed is the irregular graft thickness. The presence of striae in the stromal portion interferes with optic quality because transparency of the cornea depends on the constructive interference of light, and any distortion to the parallelism of the stromal layers may compromise optic quality. Finally, the presence of residual Descemet in the pupillary axis region in the recipient may influence optical quality.
In summary, the two main factors involved in the differing visual results among DMEK and DSEK/DSAEK are the elimination of the stromal interface in the former technique and that the technique produces an extremely thin graft. However, the premise that the architecture of the interface is a determining factor in final visual acuity may be confirmed when apparently unaltered grafts are replaced by other grafts (thicker grafts) and visual acuity improves.
Graft thickness may influence visual acuity at high values, but not in the most commonly obtained range (100-200 micrometers)41. It is therefore unclear whether thinner tissues result in better vision. In reality, the presence of any stromal interface may interfere with vision in such a way that thickness would not be the main issue.16 Therefore, the dissection of ultrathin grafts may be unnecessary.
In patients who have undergone DSEK/DSAEK, there is no compensation between anterior and posterior surface aberrations of the cornea, which is partly because of parallelism. In deep anterior penetrating lamellar transplants, this compensation is maintained. In endothelia with residual stroma, compensation of high-order optical aberrations does not occur. In addition, these aberrations may increase by 20% because of the posterior surface of the transplant. However, irregularities on the anterior surface of the cornea would be the main cause of the aberration. The difference in refraction indices between the anterior portion of the cornea and air is greater than that between the posterior portion of the cornea and the aqueous humor; once again, this would explain the importance of the anterior surface in the development of aberrations. Current equipment is limited in its ability to distinguish between the influences of each portion of the cornea on the aberrations that develop.
The donor graft surface may be another variable influencing the final visual result. Smoother grafts theoretically create a better optical interface. Therefore, it is assumed that the corneal surface dissected with a microkeratome would be smoother than one dissected with spatulas and would theoretically produce better final visual acuity.46 This theory was not proven by Prince, who obtained similar visual acuity results over 6 months in a comparison between DSEK and DSAEK. In the case of DSAEK, visual recovery was faster only in the first month; there was a lower rate of graft perforation during preparation. Stromal remodeling in the months following the use of DSEK may explain the similar visual acuity result after 6 months. Average visual acuity after DSAEK was 0.5, whereas acuity of ≥0.8 is not uncommon after DSEK through the use of grafts obtained using the technique and the correct instruments.
CONCLUSION
It is extremely important to foster further research to better understand the importance of the existing interface in endothelial transplants in the quest to increasingly improve visual quality because of these transplants. With more research, we will better understand why transplants with satisfactory anatomical results are sometimes not the same in terms of visual quality.
REFERENCES
1. Kaufman PLPLA, A.; Adler, F.H. Adler's physiology of the eye. 11 ed. ed: Elsevier; 2011.
2. Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human corneal anatomy redefined: a novel pre-Descemet's layer (Dua's layer). Ophthalmology. 013;120(9):1778-85. http://dx.doi.org/10.1016/j.ophtha.2013.01.018 PMid:23714320.
3. Straiko MD, Shamie N, Terry MA. Endothelial keratoplasty: past, present, and future directions. International ophthalmology clinics. 010;50(3):123-35. http://dx.doi.org/10.1097/IIO.0b013e3181e24746 PMid:20611023.
4. Mau K. What DSAEK is going on? An alternative to penetrating keratoplasty for endothelial dysfunction. Optometry. 2009;80(9):513-23. http://dx.doi.org/10.1016/j.optm.2008.11.010 PMid:19716079.
5. Talajic JC, Straiko MD, Terry MA. Descemet's stripping automated endothelial keratoplasty: then and now. International ophthalmology clinics. 2013;53(2):1-20. http://dx.doi.org/10.1097/IIO.0b013e31827eb6ba PMid:23470585.
6. Grottone GT, Pereira NC, Gomes JA. Endothelial keratoplasty: evolution and horizons. Arquivos brasileiros de oftalmologia. 2012;75(6):439-46. http://dx.doi.org/10.1590/S0004-27492012000600016 PMid:23715152.
7. Banitt MR, Chopra V. Descemet's stripping with automated endothelial keratoplasty and glaucoma. Current opinion in ophthalmology. 2010;21(2):144-9. http://dx.doi.org/10.1097/ICU.0b013e3283360b95 PMid:20040871.
8. Anshu A, Price MO, Tan DT, Price FW, Jr. Endothelial keratoplasty: a revolution in evolution. Survey of ophthalmology. 2012;57(3):236-52. http://dx.doi.org/10.1016/j.survophthal.2011.10.005 PMid:22516537.
9. Price MO, Price FW, Jr. Endothelial keratoplasty - a review. Clinical & experimental ophthalmology. 2010;38(2):128-40. http://dx.doi.org/10.1111/j.1442-9071.2010.02213.x PMid:20398103.
10. Giebel AW. DMEK: where less is more. International ophthalmology clinics. 2013;53(1):1-14. http://dx.doi.org/10.1097/IIO.0b013e31827744c4 PMid:23221881.
11. Almeida HG, Souza ACD de. Perfil epidemiológico de pacientes na fila de transplante de córnea no estado de Pernambuco - Brasil. Rev. bras.oftalmol. 2014; 73(1): 28-32. http://dx.doi.org/10.5935/0034-7280.20140006
12. Rose L, Kelliher C, Jun AS. Endothelial keratoplasty: historical perspectives, current techniques, future directions. Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 2009;44(4):401-5. http://dx.doi.org/10.3129/i09-090 PMid:19606160.
13. Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK- the thinner the better? Current opinion in ophthalmology. 2009;20(4):299-307. http://dx.doi.org/10.1097/ICU.0b013e32832b8d18 PMid:19417653.
14. Price MO, Price FW, Jr. Descemet's stripping with endothelial keratoplasty: comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology. 2006;113(11):1936-42. http://dx.doi.org/10.1016/j.ophtha.2006.05.034 PMid:16935344.
15. Shinton AJ, Tsatsos M, Konstantopoulos A, Goverdhan S, Elsahn AF, Anderson DF, et al. Impact of graft thickness on visual acuity after Descemet's stripping endothelial keratoplasty. The British journal of ophthalmology. 2012;96(2):246-9. http://dx.doi.org/10.1136/bjophthalmol-2011-300462 PMid:22028474.
16. Taravella MJ, Shah V, Davidson R. Ultrathin DSAEK. International ophthalmology clinics. 2013;53(2):21-30. http://dx.doi.org/10.1097/IIO.0b013e31827823a8 PMid:23470586
17. Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin descemet's stripping automated endothelial keratoplasty with the microkeratome double-pass technique: two-year outcomes. Ophthalmology. 2013;120(6):1186-94. http://dx.doi.org/10.1016/j.ophtha.2012.11.030 PMid:23466268.
18. Yoeruek E, Bayyoud T, Hofmann J, Szurman P, Bartz-Schmidt KU. Comparison of pneumatic dissection and forceps dissection in Descemet membrane endothelial keratoplasty: histological and ultrastructural findings. Cornea. 2012;31(8):920-5. http://dx.doi.org/10.1097/ICO.0b013e31823f7870 PMid:22511023.
19. Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30(12):1382-6. http://dx.doi.org/10.1097/ICO.0b013e31821ddd25 PMid:21993468.
20. Feng MT, Price MO, Price FW, Jr. Update on Descemet membrane endothelial keratoplasty (DMEK). International ophthalmology clinics. 2013;53(2):31-45. http://dx.doi.org/10.1097/IIO.0b013e31827822b9 PMid:23470587.
21. Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153(6):1082-90.e2. http://dx.doi.org/10.1016/j.ajo.2011.12.012
22. Maier AK, Gundlach E, Gonnermann J, Klamann MK, Eulufi C, Bertelmann E, et al. Fellow Eye Comparison of Descemet Membrane Endothelial Keratoplasty and Penetrating Keratoplasty. Cornea. 2013. http://dx.doi.org/10.1097/ICO.0b013e31829dd816 PMid:23928950.
23. Armour RL, Ousley PJ, Wall J, Hoar K, Stoeger C, Terry MA. Endothelial keratoplasty using donor tissue not suitable for full-thickness penetrating keratoplasty. Cornea. 2007;26(5):515-9. PMid:17525642.
24. Phillips PM, Terry MA, Shamie N, Chen ES, Hoar KL, Stoeger C, et al. Descemet's stripping automated endothelial keratoplasty (DSAEK) using corneal donor tissue not acceptable for use in penetrating keratoplasty as a result of anterior stromal scars, pterygia, and previous corneal refractive surgical procedures. Cornea. 2009;28(8):871-6. http://dx.doi.org/10.1097/ICO.0b013e318199f8d7 PMid:19654530.
25. Moshirfar M, Khalifa YM, Davis D, Fenzl CR, Espandar L, Chang JC, et al. Descemet stripping automated endothelial keratoplasty using donor corneas with previous laser in situ keratomileusis or photorefractive keratectomy: a case series and donor cap histopathology. Cornea. 2012;31(5):533-7. http://dx.doi.org/10.1097/ICO.0b013e31820142be PMid:21993455.
26. Khor WB, Teo KY, Mehta JS, Tan DT. Descemet stripping automated endothelial keratoplasty in complex eyes: results with a donor insertion device. Cornea. 2013;32(8):1063-8. http://dx.doi.org/10.1097/ICO.0b013e31828321f8 PMid:23449486.
27. Javadi MA, Feizi S, Jafari R, Mirbabaee F, Ownagh V. Descemet Stripping Automated Endothelial Keratoplasty in Fuchs' Endothelial Dystrophy versus Pseudophakic Bullous Keratopathy. J Ophthalmic Vis Res. 2016 Oct-Dec; 11(4): 372–378. http://dx.doi.org/10.4103/2008-322X.194073. PMCID: PMC5139549.
28. Price MO, Price FW, Jr., Trespalacios R. Endothelial keratoplasty technique for aniridic aphakic eyes. Journal of cataract and refractive surgery. 2007;33(3):376-9. http://dx.doi.org/10.1016/j.jcrs.2006.10.052 PMid:17321384.
29. Busin M, Beltz J, Scorcia V. Descemet-stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Archives of ophthalmology. 2011;129(9): 1140-6. http://dx.doi.org/10.1001/archophthalmol.2011.114 PMid:21555597.
30. Anwar HM, El Danasoury A, Hashem A. Descemet's stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Clinical ophthalmology. 2012;6:159-63. http://dx.doi.org/10.2147/OPTH.S28405 PMid:22291459 PMCid:PMC3267538.
31. Hashemi H, Ghaffari R, Mohebi M. Posterior lamellar keratoplasty (DSAEK) in Peters anomaly. Cornea. 2012;31(10):1201-5. http://dx.doi.org/10.1097/ICO.0b013e31825697a4 PMid:22790185.
32. Covert DJ, Koenig SB. Descemet stripping and automated endothelial keratoplasty (DSAEK) in eyes with failed penetrating keratoplasty. Cornea. 2007;http://dx.doi.org/10.1097/ICO.0b013e31805fc38f26(6):692-6. PMid:17592318.
33. Straiko MD, Terry MA, Shamie N. Descemet stripping automated endothelial keratoplasty under failed penetrating keratoplasty: a surgical strategy to minimize complications. American journal of ophthalmology. 2011;151(2):233-7 e2. http://dx.doi.org/10.1016/j.ajo.2010.08.017
34. Graue-Hernandez EO, Zuniga-Gonzalez I, Hernandez-Camarena JC, Jaimes M, Chirinos-Saldana P, Navas A, et al. Tectonic DSAEK for the Management of Impending Corneal Perforation. Case reports in ophthalmological medicine. 2012;2012:916528. http://dx.doi.org/10.1155/2012/916528 PMid:23259100 PMCid:PMC3521400.
35. Dirisamer M, Ham L, Dapena I, van Dijk K, Melles GR. Descemet membrane endothelial transfer: "free-floating" donor Descemet implantation as a potential alternative to "keratoplasty". Cornea. 2012;31(2):194-7. http://dx.doi.org/10.1097/ICO.0b013e31821c9afc PMid:22146548.
36. Dirisamer M, Yeh RY, van Dijk K, Ham L, Dapena I, Melles GR. Recipient endothelium may relate to corneal clearance in descemet membrane endothelial transfer. American journal of ophthalmology. 2012;154(2):290-6 e1. DOI: http://dx.doi.org/10.1016/j.ajo.2012.02.032
37. Lim LS, Aung HT, Aung T, Tan DT. Corneal imaging with anterior segment optical c oherence tomography for lamellar keratoplasty procedures. American journal of ophthalmology. 2008;145(1):81-90. http://dx.doi.org/10.1016/j.ajo.2007.08.019 PMid:18028862.
38. Tong CM, Melles GR. Where is endothelial keratoplasty going: from Descemet stripping (automated) endothelial keratoplasty to Descemet membrane endothelial keratoplasty to Descemet membrane endothelial transfer? Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 2012;47(3):197-200. http://dx.doi.org/10.1016/j.jcjo.2012.04.009 PMid:22687292.
39. Dirisamer M, Parker J, Naveiras M, Liarakos VS, Ham L, van Dijk K, et al. Identifying causes for poor visual outcome after DSEK/DSAEK following secondary DMEK in the same eye. Acta ophthalmologica. 2013;91(2):131-9. http://dx.doi.org/10.1111/j.1755-3768.2012.02504.x PMid:22989010.
40. Koh S, Maeda N, Nakagawa T, Nishida K. Quality of vision in eyes after selective lamellar keratoplasty. Cornea. 2012;31 Suppl 1:S45-9. http://dx.doi.org/10.1097/ICO.0b013e318269c9cd PMid:23038035.
41. Terry MA, Straiko MD, Goshe JM, Li JY, Davis-Boozer D. Descemet's stripping automated endothelial keratoplasty: the tenuous relationship between donor thickness and postoperative vision. Ophthalmology. 2012;119(10):1988-96. http://dx.doi.org/10.1016/j.ophtha.2012.05.021 PMid:22817831.
42. Yamaguchi T, Ohnuma K, Tomida D, Konomi K, Satake Y, Negishi K, et al. The contribution of the posterior surface to the corneal aberrations in eyes after eratoplasty. Investigative ophthalmology & visual science. 2011;52(9):6222-9. http://dx.doi.org/10.1167/iovs.11-7647 PMid:21724912.
43. Koh S, Maeda N, Nakagawa T, Higashiura R, Saika M, Mihashi T, et al. Characteristic higher-order aberrations of the anterior and posterior corneal surfaces in 3 corneal transplantation techniques. American journal of ophthalmology. 2012;153(2):284-90 e1. DOI: http://dx.doi.org/10.1016/j.ajo.2011.06.027
44. Sarayba MA, Ignacio TS, Binder PS, Tran DB. Comparative study of stromal bed quality by using mechanical, IntraLase femtosecond laser 15- and 30-kHz microkeratomes. Cornea. 2007;26(4):446-51. http://dx.doi.org/10.1097/ICO.0b013e318033e7cc PMid:17457194.
45. Marian A, Nada O, Legare F, Meunier J, Vidal F, Roy S, et al. Smoothness assessment of corneal stromal surfaces. Journal of cataract and refractive surgery. 2013;39(1):118-27. http://dx.doi.org/10.1016/j.jcrs.2012.08.050 PMid:23128030.
46. Lombardo M, De Santo MP, Lombardo G, Schiano Lomoriello D, Desiderio G, Ducoli P, et al. Surface quality of femtosecond dissected posterior human corneal stroma investigated with atomic force microscopy. Cornea. 2012;31(12):1369-75. http://dx.doi.org/10.1097/ICO.0b013e31823f774c PMid:22262224.
47. Ham L, Dapena I, van der Wees J, Melles GR. Secondary DMEK for poor visual outcome after DSEK: donor posterior stroma may limit visual acuity in endothelial keratoplasty. Cornea. 2010;29(11):1278-83. http://dx.doi.org/10.1097/ICO.0b013e3181cda01a PMid:20697285.
48. van der Meulen IJ, van Riet TC, Lapid-Gortzak R, Nieuwendaal CP, van den Berg TJ. Correlation of straylight and visual acuity in long-term follow-up of manual descemet stripping endothelial keratoplasty. Cornea. 2012;31(4):380-6. http://dx.doi.org/10.1097/ICO.0b013e31823f8ab7 PMid:22262226.



Fonte de financiamento: declaram não haver.
Conflito de interesses: declaram não haver.
Received on:
October 19, 2015.
Accepted on:
November 13, 2015.