Isaac Carvalho de Oliveira Ramos1; Guilherme Ribeiro2; Andréia Peltier de Queiroz Urbano de Souza3
DOI: 10.17545/eOftalmo/2017.94
ABSTRACT
Technological advances in biometric calculation, surgical techniques, phacoemulsification equipment, and intraocular lenses (IOLs) have made cataract surgery essentially a refractive procedure, which aims not only to gain more postoperative lines of visual acuity but also better visual quality. When considering visual acuity, it is essential to go beyond the Snellen chart and use aberrometry concepts. This update by the Literature Review Group of the Brazilian Society for Cataract and Refractive Surgery aims to emphasize the importance of preoperative aberrometry examinations in patients who are scheduled to undergo cataract surgery because of its ability to optimize surgical planning and to enable a better selection of IOLs.
Keywords: Cataract; Aberrometry; Visual acuity;
RESUMO
Com a evolução tecnológica dos cálculos biométricos, das técnicas cirúrgicas, dos equipamentos de facoemulsificação e das lentes intraoculares (LIOs), a cirurgia de catarata tornou-se um procedimento essencialmente refrativo, no qual se almeja atingir no pós-operatório não somente ganho de linhas de visão, mas de qualidade visual. Quando se fala de qualidade visual, deve-se ir além da tabela de Snellen e adentrar os conceitos da Aberrometria. O Grupo de Revisão da Literatura da Sociedade Brasileira de Catarata e Cirurgia Refrativa (ABCCR/BRASCS), com esta atualização sobre o tema, pretende ressaltar a importância do exame de Aberrometria pré-operatório para o paciente que será submetido à Cirurgia de Catarata, pela sua grande utilidade para a otimização do planejamento cirúrgico e melhor escolha da lente intraocular.
Palavras-chave: Catarata; Aberrometria; Acuidade visual;
INTRODUCTION
Technological advances in biometric calculation, surgical techniques, phacoemulsification equipment, and intraocular lenses (IOLs) have made cataract surgery essentially a refractive procedure, which aims not only to gain more postoperative lines of visual acuity but also better visual quality. When considering visual acuity, it is essential to go beyond the Snellen chart and use aberrometry concepts.
Aberrometry is the field of study concerned with optical aberrations, which can be categorized into chromatic and monochromatic.
Chromatic aberrations occur because each color has a different wavelength and hence a different refractive index. Thus, each color of the visible spectrum is refracted differently (Figure 1).
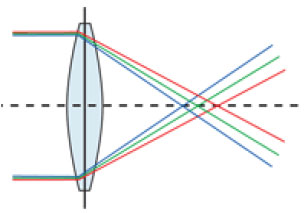
Short-wavelength colors, such as green, are more anteriorly focused (on the cornea and lens) compared with long-wavelength colors, such as red, which are more posteriorly focused. Studies have shown that there can be ≤ 2 D of chromatic difference within the visual spectrum, which clearly affects the patient’s visual quality.1,2 Although it is known that such a difference exists, no equipment that can sensitively evaluate its influence on visual function has yet been created. However, some methods are currently used to improve chromatic dispersion in IOLs; for example, the Tecnis Symfony® IOL (Abbott Medical Optics) uses chromatic aberration control technology to reduce the dispersion of different wavelengths, thereby improving postoperative visual acuity (Figure 2).3
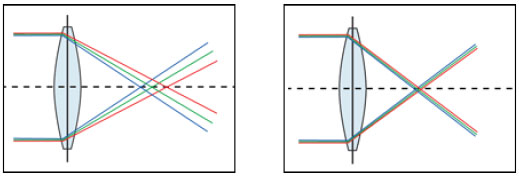
In contrast, monochromatic aberrations are already well studied, and their influence on visual acuity must be measured in cataract patients during both the pre- and postoperative periods. Monochromatic aberrations can be categorized into lower-order and higher-order aberrations. The former includes refractive errors, such as myopia (myopic defocus) and hyperopia (hyperopic defocus), as well as astigmatism. Although higher-order aberrations, such as coma, trefoil, and spherical aberrations, are a small part of refractive errors in normal eyes, they can negatively influence visual quality, particularly in terms of mesopic contrast.4
Because ocular aberrations are directly proportional to the pupil size,5 reduction in the pupil size results in the iris becoming a real barrier to more peripheral and aberrant light beams, allowing only more central aberrations to be perceived by the retina. In larger pupils, peripheral light , which presents higher-order aberrations, lead to light dispersion and therefore more blurring of the retinal image (Figure 3).6 (Figura 3). The preoperative evaluation of such aberrations is a determining factor for the correct surgical planning ad selection of IOL to be implanted.
IOL Selection Based on Aberrometry
The selection of IOL to be implanted is currently a very important step in cataract surgery because of the wide variety in the available models and technologies. Notably, despite the sophistication of technological advances, the most modern or advanced IOL is not necessarily the one that will provide the best visual outcomes.
Using aberrometry to aid in this task allows for two approaches:
– the selection between spherical and aspheric IOL
– indication or contraindication for multifocal lenses
Spherical vs. Aspheric IOLs
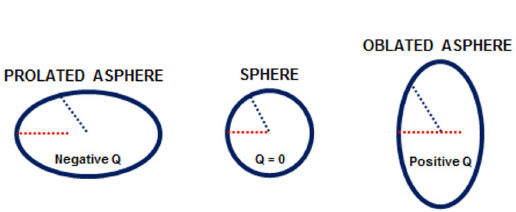
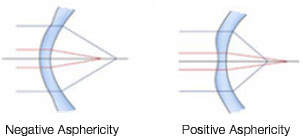
Asphericity (Q value) is a geometric measure of ellipsoid surfaces. It represents the change in the curvature of an ellipsoid surface from the center toward the edges. Thus, because a perfect sphere has equal radii and the same curvature from the center to the edges, it has zero asphericity (Q = 0). In contrast, an elliptical object with shorter central radii (i.e., a more curved center) than peripheral radii is described as a prolate sphere, and it has negative asphericity (negative Q value). In addition, an elliptical object with longer central radii (a flatter center) than peripheral radii is described as an oblate sphere, and it has positive asphericity (positive Q value) (Figure 4).
Because the human cornea is not perfectly spherical in shape and has different curvature radii, its Q value is not zero. Thus, a normal cornea, which the center is more curved than its edges, is described as prolate with negative asphericity (negative Q value). After photoablation for myopia, the postoperative cornea will have a flatter center than edges; therefore, it will become oblate with positive asphericity (positive Q value) (Figure 5).
Every lens has some degree of asphericity, and its Q value is directly proportional to the induction of a higher-order monochromatic aberration called spherical aberration. This is described as the difference between light refraction at the center and at the edges of the lens due to the angle of incidence of light beams on the lens surface. Therefore, light beams refracted at the center will be focused on a given point and peripheral beams will be reflected on a different point (Figure 6). Every optical system with a spherical aberration suffers from image distortion and a consequent loss of visual quality.

The human cornea has an average asphericity of −0.23 µm. This peculiar shape induces an average positive spherical aberration of +0.28 µm.7 Despite this positive spherical aberration of the cornea, the lens of a young patient generates a negative aspherical aberration when accommodating. This practically compensates the corneal aberration, thereby generating a balance and consequently good nearsight.8
As people age, the cornea either retains its shape or tends to acquire a slightly negative aberration. However, the natural aging of the lens results in increased volume and thickness of both its central and peripheral portions. By losing its plasticity, the lens tends to generate a positive spherical aberration. Thus, as people age, presbyopia occurs: the total positive spherical aberration of the eye is increased and the quality of nearsight is impaired due to the lack of a balance between the cornea and the previously accommodating lens (Figure 7).9
The first type of IOL to be manufactured was a spherical IOL. In this type of lens, beams that pass through the edges converge anteriorly to paracentral beams, creating a positive spherical aberration.
Thus, using the first developed spherical IOLs, the contrast sensitivity of pseudophakic patients was not found to differ significantly from that of phakic patients of the same age who had cataracts. In other words, when spherical IOLs were implanted, postoperative visual acuity did not meet expectations in most cases.10 This outcome was because the cornea had a positive spherical aberration and spherical IOLs that were implanted also had a positive spherical aberration, thereby introducing a highly positive higher-order aberration into the total optical system and consequently producing a loss in visual acuity.
In an attempt to mimic the spherical aberration of a young lens, Holladay proposed aspheric IOLs in 2002. This type of lens has a specific geometrical design in terms of both its posterior face and edges. This design attempts to change the curvature radii of the lens from the center toward the edges so that light beams are focused on the same point, thereby improving visual acuity. Because different designs of aspheric IOLs are available, lenses with either negative or zero (neutral) spherical aberration may be obtained.
Therefore, the basic difference between spherical and aspheric lenses is that the entire surface of a spherical lens has the same curvature, causing peripheral light beams to be refracted differently from centrally refracted beams (models with positive spherical aberration). In contrast, the anterior and/or posterior surface of the edge of an aspheric lens is curved differently from its center to compensate that difference, which represents models with either negative or zero spherical aberration (Figure 8).
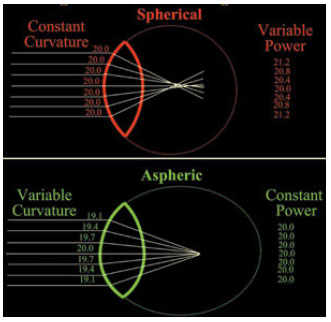
The most modern designs of aspheric IOLs seek to induce a negative spherical aberration to partly compensate corneal spherical aberrations and improve patient’s visual performance, particularly in terms of mesopic contrast.11
In normal corneas, aspheric lenses were found to provide better contrast sensitivity than spherical lenses, particularly in mesopic conditions when subjected to glare situations (Figure 9).12
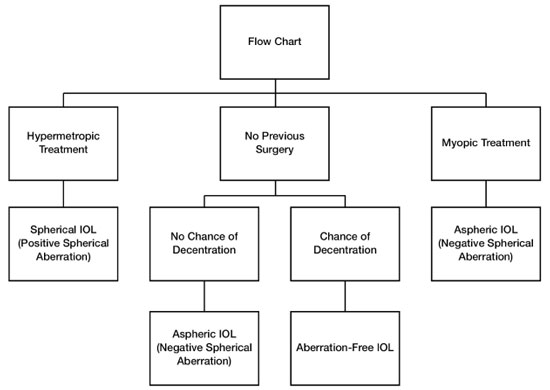
However, aspheric lenses are not indicated for all eyes. Patients who have undergone refractive surgery (RK, LASIK, and/or PRK) are aging and now undergoing cataract surgery. Studies have demonstrated that excimer laser surface surgery leads to a modification in the anterior surface that changes the cornea’s natural asphericity. A positive spherical aberration is induced in patients who undergo myopia treatment in which the center of their cornea is flattened, whereas a negative spherical aberration is induced in patients who undergo hyperopia treatment in which the center becomes more curved.13
Thus, aspheric IOLs with negative spherical aberrations are recommended to compensate for the positive spherical aberration induced by photoablation in patients who have undergone myopia treatment. On the other hand, for patients who have undergone hyperopia treatment, the best choice is a classic spherical IOL because it induces a positive spherical aberration that can compensate for the negative spherical aberration resulting from photoablation (Figure 9).
In cases in which there is a possibility of zonular fragility or other alterations that may predispose the IOL to be displaced from the center, aspheric IOLs with zero (neutral) spherical aberration are recommended. It is known that when aspheric IOLs with negative spherical aberration are displaced from the center, a high degree of the higher-order aberration called coma is induced, which causes a loss of visual quality (Figure 9).14,15
Contraindication for Multifocal IOLs Based on Aberrometry
The literature describes patients complaining of optical phenomena (halos, glare, or diminished contrast) after undergoing cataract surgery, particularly when multifocal IOLs are used. These phenomena occur because of splitting of the image with loss of contrast sensitivity, regardless of whether the lens technology is refractive or diffractive. Thus, the indication for multifocal IOLs involves several aspects of the patient. Few patients, such as very demanding patients with high expectations, airplane pilots, and professionals who often drive at night, are contraindicated for multifocal IOLs.
In aberrometry, common sense dictates a careful preoperative evaluation of higher-order aberrations in patients who are candidates for multifocal IOLs because patients who already have a high degree of such aberrations complain more often of postoperative dysphotopsia, glare, and halos, which may cause them to fail to adapt to the multifocal IOL and therefore require lens replacement.16
According to the literature, the main higher-order aberration that causes these types of complaints is coma (asymmetrical aberration). Some studies have suggested a cutoff coma measurement of 0.32 or 0.33 µm to contraindicate a multifocal IOL.16,17
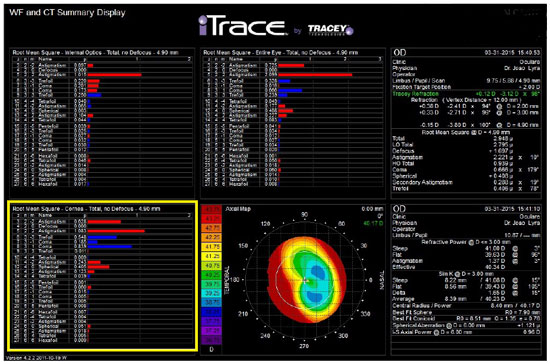
When studying preoperative ocular aberrations in cataract surgery, we can benefit from information such as that from the patient in Figure 10 who underwent keratorefractive surgery and exhibited a coma aberration of 0.666 µm, which would probably hamper the adaptation to a multifocal IOL. A spherical aberration of +1.121 µm resulting from a secondary central flattening during the previous myopia treatment can also be observed in the figure. This is an ideal patient in whom a monofocal aspheric IOL with a negative spherical aberration can be used.
A Novel Technology (ORA® )
ORA® (Optiwave Refractive Analysis; WaveTec Vision Systems Inc., Aliso Viejo, CA, USA) is an intraoperative aberrometry analysis platform. It was developed to further increase biometric precision in cataract surgery using aberrometry to yield more predictable and precise results for the effective power of the lens.
Wavefront technologies, such as Hartmann–Shack, use mathematical principles to capture and analyze a wavefront. However, such technologies are not suitable for use in cataract surgery because the devices are too large to be incorporated into the surgical microscope. In addition, these technologies are limited to an average refractive range of −10 D to +8 D, whereas a typical cataract patient’s eye has a refraction of +13 D. ORA® uses a novel form of wavefront analysis known as Talbot–Moiré interferometry.18 which allows the equipment to be compact, light, and directly attachable to the surgical microscope, without interfering with the surgeon’s workspace. It also covers a refractive range of −20 D to +20 D.
The refraction measurements using this equipment can be performed in phakic, aphakic, or pseudophakic modes. Aphakic-mode refractions are primarily developed to help correct the spherical and cylindrical values of IOLs. Pseudophakic readings are obtained to confirm the refractive error programmed in the preoperative phase, to help position toric IOLs, and to help with degree measurements in limbal relaxing incisions.
Studies have demonstrated that readings from this equipment may be affected by corneal edema and some types of blepharostat.19 The best reproducible measurements are obtained in the aphakic mode using a viscoelastic material.20 Nevertheless, a recent study by Maket et al. tested six different viscoelastic materials and their possible interference in intraoperative aberrometry biometric readings. The authors observed that two of the six materials produced a biometric difference of ≤0.50 compared with BSS. This biometric discrepancy can be explained by the different refractive indices of the materials. Thus, the authors have suggested a careful evaluation of the measured values and attempts to correct the lens choice nomogram if necessary.21
Despite these limitations, studies have demonstrated that using intraoperative aberrometry increases the odds of obtaining a residual refractive astigmatism of ≤0.50 D by 2.4 times compared with that by conventional methods. In additional, these studies have demonstrated that the use of intraoperative aberrometry significantly changes the decision-making process during surgery, with reported change in cylindrical power in 24% of cases, in spherical power in 25% of cases, and in rotations in 92% of cases of toric lens implantation.22 Furthermore, studies have demonstrated that the use of this technology may provide superior refractive results in patients who have undergone prior corneal refractive surgery (LASIK or PRK) compared with those by conventional methods for toric lenses.23,24
CONCLUSION
In light of these considerations, the preoperative aberrometry examination of patients who are scheduled to undergo cataract surgery is currently considered extremely useful for optimizing surgery planning and selecting the best IOL. Taking these preoperative measures can lead to better postoperative visual acuity in patients and can help avoid patient dissatisfaction resulting from the influence of previous ocular aberrations in the photorefractive outcome.
REFERENCES
1. Jankov M, Mrochen M, Schor P, Chamon W, Seiler T. JankovMirko, Mrochen Michael, Schor Paulo, Chamon Wallace, Seiler Theo. Frentes de ondas (wavefronts) e limites da visão humana Parte 1: fundamentos. Arq Bras Oftalmol 2002;65(6):679–84. https://doi.org/10.1590/S0004-27492002000600016
2. Zhao H, Mainster MA. The effect of chromatic dispersion on pseudophakic optical performance. Br J Ophthalmol 2007;91(9):1225–9. https://doi.org/10.1136/bjo.2007.118745
3. Bethke W. IOL alternatives to multifocality. Review of Ophthalmology 2015;22(1):32. Disponível em: https://www.reviewofophthalmology.com/article/iol-alternatives-to-multifocality
4. Oshika T, Okamoto C, Samejima T, Tokunaga T, Miyata K. Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes. Ophthalmology 2006;113(10):1807–12. https://doi.org/10.1016/j.ophtha.2006.03.061
5. Wang Y, Zhao K, Jin Y, Niu Y, Zuo T. Changes of higher order aberration with various pupil sizes in the myopic eye. J Refract Surg 2003;19(2 Suppl):S270–4. PMID:12699188
6. Urbano et al. Medidas da qualidade da imagem. Wavefront Cirurgia Personalizada. Rio de Janeiro. Cultura Médica, 2006.
7. Wang L, Dai E, Koch DD, Nathoo A. Optical aberrations of the human anterior cornea. J Cataract Refract Surg 2003;29(8):1514–21. https://doi.org/10.1016/S0886-3350(03)00467-X
8. Artal P, Guirao A, Berrio E, Williams DR. Compensation of corneal aberrations by the internal optics in the human eye. J Vis 2001;1(1):1–8. https://doi.org/10.1167/1.1.1
9. Amano S, Amano Y, Yamagami S, Miyai T, Miyata K, Samejima T, Oshika T. Age-related changes in corneal and ocular higher-order wavefront aberrations. Am J Ophthalmol 2004;137(6):988–92. https://doi.org/10.1016/j.ajo.2004.01.005
10. Nio YK, Jansonius NM, Fidler V, Geraghty E, Norrby S, Kooijman AC. Age-related changes of defocus-specific contrast sensitivity in healthy subjects. Ophthalmic Physiol Opt 2000;20(4):323–34. https://doi.org/10.1016/S0275-5408(99)00103-9
11. Holladay JT, Piers PA, Koranyi G, van der Mooren M, Norrby NE. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg 2002;18(6):683–91. PMID:12458861
12. Kershner RM. Retinal image contrast and functional visual performance with aspheric, silicone, and acrylic intraocular lenses. Prospective evaluation. J Cataract Refract Surg 2003;29(9):1684–94. https://doi.org/10.1016/S0886-3350(03)00523-6
13. Bottos KM, Leite MT, Aventura-Isidro M, Bernabe-Ko J, Wongpitoonpiya N, Ong-Camara NH, Purcell TL, Schanzlin DJ. Corneal asphericity and spherical aberration after refractive surgery. J Cataract Refract Surg 2011;37(6):1109–15. https://doi.org/10.1016/j.jcrs.2010.12.058
14. Altmann GE, Nichamin LD, Lane SS, Pepose JS. Optical performance of 3 intraocular lens designs in the presence of decentration. J Cataract Refract Surg 2005;31(3):574–85. https://doi.org/10.1016/j.jcrs.2004.09.024 PMID:15811748
15. Nanavaty MA, Spalton DJ, Marshall J. Effect of intraocular lens asphericity on vertical coma aberration. J Cataract Refract Surg 2010 Feb;36(2):215–21. https://doi.org/10.1016/j.jcrs.2009.08.024
16. Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, Hoffman R, Little B, Mamalis N, Oetting T, Serafano D, Talley-Rostov A, Vasavada A, Yoo S; ASCRS Cataract Clinical Committee. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg 2014;40(2):313–22. https://doi.org/10.1016/j.jcrs.2013.12.011
17. Hamza I, Aly MG, Hashem KA. Multifocal IOL dissatisfaction in patients with high coma aberrations. Presented at: ASCRS Symposium on Cataract, IOL and Refractive Surgery; March 27, 2011; San Diego, CA.
18. Wiley WF, Bafna S. Intra-operative aberrometry guided cataract surgery. Int Ophthalmol Clin 2011;51(2):119–29. https://doi.org/10.1097/IIO.0b013e31820f226d
19. Stringham J, Pettey J, Olson RJ. Evaluation of variables affecting intraoperative aberrometry. J Cataract Refract Surg 2012 Mar;38(3):470–4. https://doi.org/10.1016/j.jcrs.2011.09.039
20. Huelle JO, Katz T, Druchkiv V, Pahlitzsch M, Steinberg J, Richard G et al. First clinicial results on the feasibility, quality and reproducibility of aberrometry-based intraoperative refraction during cataract surgery. Br J Ophthalmol 2014;98(11):1484–91. https://doi.org/10.1136/bjophthalmol-2013-304786
21. Masket S, Fram NR, Holladay JT. Influence of ophthalmic viscosurgical devices on intraoperative aberrometry. J Cataract Refract Surg 2016;42(7):990–4. https://doi.org/10.1016/j.jcrs.2016.04.022
22. Hatch KM, Woodcock EC, Talamo JH. Intraocular lens power selection and positioning with and without intraoperative aberrometry. J Refract Surg 2015 Apr;31(4):237–42. https://doi.org/10.3928/1081597X-20150319-03
23. Canto AP, Chhadva P, Cabot F, Galor A, Yoo SH, Vaddavalli PK, Culbertson WW. Comparison of IOL power calculation methods and intraoperative wavefront aberrometer in eyes after refractive surgery. J Refract Surg 2013;29(7):484–9. https://doi.org/10.3928/1081597X-20130617-07
24. Ianchulev T, Hoffer KJ, Yoo SH, Chang DF, Breen M, Padrick T, Tran DB. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology 2014;121(1):56–60. https://doi.org/10.1016/j.ophtha.2013.08.041



Received on:
February 27, 2017.
Accepted on:
March 11, 2017.