Kenzo Hokazono; Ana Bárbara Dias Lopes Urzedo
DOI: 10.17545/eOftalmo/2024.0003
Este artigo pertence à Edição Especial Edição especial Neuro
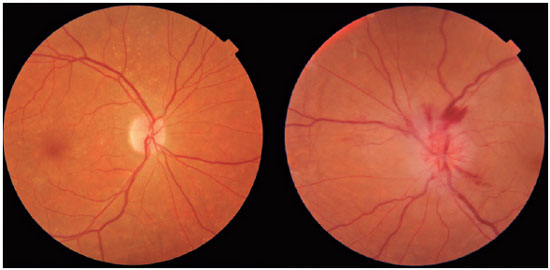
Anterior ischemic optic neuropathy (AION) is the most common cause of acute optic neuropathy in patients older than age 50 and is caused by ischemia of the short posterior ciliary arteries. Risk factors include systemic arterial hypertension (SAH), diabetes mellitus (DM), and a crowded optic nerve head. The diagnosis is clinical and the condition has a typical clinical manifestation: sudden, painless, unilateral loss of visual acuity (VA), mainly in older adults and upon waking. On ophthalmological examination, disc edema and peripapillary hemorrhage are apparent (Figure 1), in addition to relative afferent pupillary defect (RAPD). AION can be classified as arteritic (A-AION) or nonarteritic (NA-AION), which have important epidemiological, clinical, and therapeutic differences. Therefore, when faced with optic neuropathy, performing a differential diagnosis to identify the specific etiology is essential1. Several etiologies must be considered, such as inflammatory, compressive, nutritional-toxic, heredodegenerative, infectious, and vascular causes1,2.
For differential diagnosis of the several causes of optic neuropathies, clinical reasoning becomes essential. In cases of optic neuropathies that present with sudden vision loss, like AION, compressive, nutritional, toxic, and heredodegenerative causes are less likely because they tend to induce chronic and indolent loss of vision.
Inflammatory diseases are primarily caused by optic neuritis and, unlike AION, usually have a course involving subacute VA loss (within 2 or 3 days) associated with pain on extraocular movement. Furthermore, optic neuritis is mainly observed in young women and most commonly affects the retrobulbar portion of the optic nerve. It does not cause disc edema. Infectious diseases can be differentiated when they cause some degree of ocular inflammation, such as anterior chamber or vitreous reactions, in addition to systemic alterations, such as fever, arthralgia, or skin rash1,2.
In addition, DM can cause an optic neuropathy known as diabetic papillopathy. However, this affects younger patients with type 1 DM and results in only a slight loss of VA3. An association has been indicated between SAH and a subtype of central retinal vein occlusion called papillophlebitis, which also affects young patients with mild VA loss. In these cases, the patient has hyperemia, mild edema, and venous dilatation over the disc, with an increase in blind spots within the visual field and the absence of a RAPD1.
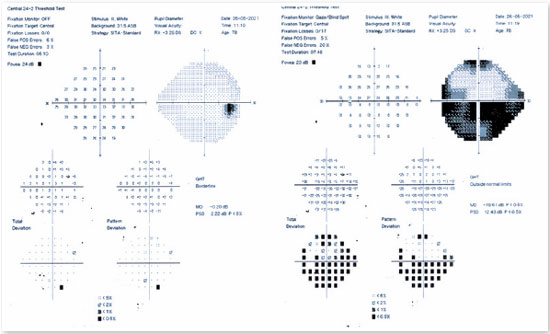
Among the complementary ophthalmological tests useful in confirming a diagnosis of AION, computerized campimetry is very important, as the defect shown is peculiar to AION and affects half of the field, indicating an altitudinal defect (Figure 2). It is important to consider that nerve compressive diseases cause diffuse field loss; toxic, nutritional, and hereditary diseases cause defects in the cecocentral region (the region that connects central vision to the blind spot); and infectious and inflammatory diseases mainly cause central defects1.
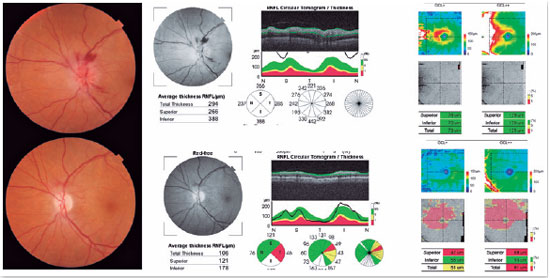
Optical coherence tomography (OCT) can help in the diagnosis of AION because, in addition to detecting sectorial disc edema (Figure 3), it can provide data that exclude other causes of optic neuropathy, such as edema and macular exudate in cases of neuroretinitis, and hyperreflectivity of the photoreceptor layer in cases of neuritis due to syphilis1.
For etiological elucidation, complete blood count and serology are helpful for the exclusion of infectious diseases such as syphilis, cat scratch disease, and Lyme disease. Fasting glucose and glycated hemoglobin levels enable the exclusion of diabetic papillopathy, while testing for SAH can aid the diagnosis of papillophlebitis. Other tests, such as antinuclear antibody tests and the purified protein derivative test, can help exclude less common diseases, such as vasculitis and tuberculosis1,2.
Imaging tests, such as computed tomography and orbital resonance, may exclude tumors and compression of the optic nerve1.
Once an AION diagnosis has been made, the clinician must determine whether the patient has the arteritic or nonarteritic form. The arteritic form, which is associated with giant cell arteritis, causes irreversible vision loss in the contralateral eye in 50% of cases and should therefore be aggressively treated with corticosteroids. C-reactive protein levels and the erythrocyte sedimentation rate are fundamental blood tests because A-AION patients present with a significant elevation of these markers. Elevations of both of these markers has 97% sensitivity in detecting the disease4. Table 1 shows characteristics that differentiate the two conditions.
No treatment has yet been found that improves VA in patients with NA-AION. Patients usually remain stable over time, with VA stabilization occurring within the first 2 months. Approximately 16%-42% of patients may recover three lines of vision. Recurrent episodes of vision loss in the same eye are uncommon after 3 months (ranging from 3% to 8%) and are more frequent in younger patients. The incidence of contralateral eye involvement ranges from 15% to 24% at 15 years5.
REFERENCES
1. Monteiro MLR, Alves MR. Neuroftalmologia. 3ª edição, Rio de Janeiro: 2013.
2. Kandeger BT, Tok O, Tok L. Clinical, demographic characteristics, and treatment protocols of optic neuropathies: Three-year follow-up experiences from a tertiary hospital in Turkey. Curr J Neurol. 2022;21(3):170–177.
3. Kovacova A, Shotliff K. Eye Problems in People with Diabetes: More than Just Diabetic Retinopathy. Pract Diabetes. 2022;39(1):34-39a.
4. Parikh M, Miller NR, Lee AG, Savino PJ, Vacarezza MN, Cornblath W, et al. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology. 2006;113(10):1842-1845.
5. Lee YC, Wang JH, Huang TL, Tsai RK. Increased risk os stroke in patients with nonarteritic anterior ischemic optic neuropathy: a nationwide retrospective cohort study. Am J Ophthalmol. 2016 Oct;170:183-189.
AUTHORS INFORMATIONS |
|
 |
» Kenzo Hokazono https://orcid.org/0000-0003-2897-6833 http://lattes.cnpq.br/6977006766618836 |
 |
» Ana Bárbara Dias Lopes Urzedo https://orcid.org/0000-0003-1113-3186 http://lattes.cnpq.br/8922699183833094 |
Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
June 6, 2023.
Accepted on:
September 13, 2023.