Francisco Emanoel Albuquerque de Souza Júnior1; Ridson Guilherme Parente de Aguiar1; Juliana de Lucena Martins Ferreira2; João Crispim Moraes Lima Ribeiro2,3
DOI: 10.17545/eOftalmo/2023.0030
ABSTRACT
OBJECTIVE: Infantile nystagmus syndrome is an eye movement disorder characterized by involuntary rhythmic oscillations of the eyes that are persistent throughout life, with a prevalence of about 0.14% in the general population. The aim of this study was to review the literature on infantile nystagmus syndrome to address diagnoses, surgical, optical, and pharmacological treatments.
METHODS: A literature review was conducted, drawing on articles from the PubMed and LILACS platforms covering the years 2000 to 2022. The exclusion criteria were: articles published before 2000, duplicate articles, case reports that are not in PubMed or LILACS, and articles that are not related to the review topic.
RESULTS: The diagnosis is clinical, but tests such as optical coherence tomography and electroretinography are necessary to determine the etiology. Gene sequencing, either conventional Sanger (FRMD7 and GPR143) or next-generation gene sequencing, can help determine the etiology. The use of glasses can reduce nystagmus in some cases. Surgical treatment improves visual performance and correct anomalous head posture. Additionally, several studies have reported favorable results of gabapentin and memantine use in patients with infantile nystagmus syndrome.
CONCLUSIONS: Although there are optical, pharmacological, and surgical treatments for childhood nystagmus syndrome, more research is needed to expand the types of treatments because treatment does not cure the disease permanently and completely.
Keywords: Congenital nystagmus; Ocular motility disorders; Eye diseases.
RESUMO
OBJETIVO: A síndrome do nistagmo infantil é um movimento constante de vai e vem dos olhos, persistente ao longo da vida, com prevalência de cerca de 0,14% na população geral. revisar a literatura sobre síndrome do nistagmo infantil, buscando abordar diagnósticos, tratamentos cirúrgicos, ópticos e farmacológicos.
MÉTODOS: Realizou-se uma revisão de literatura, elaborada com artigos das plataformas PubMed e LILACS, abrangendo os anos de 2000 a 2022. Os critérios de exclusão foram: artigos publicados antes de 2000, artigos duplicados, relatos de casos, não estar no PubMed ou LILACS e não estar relacionado ao tema da revisão.
RESULTADOS: O diagnóstico é clínico, mas exames como tomografia de coerência óptica e eletrorretinografia são necessários para determinar a etiologia. O sequenciamento genético, seja Sanger convencional (FRMD7 e GPR143) ou sequenciamento de genes de próxima geração, pode ajudar a determinar a etiologia. O uso de óculos pode reduzir o nistagmo em alguns casos. O tratamento cirúrgico visa melhorar o desempenho visual e corrigir a postura anômala da cabeça. Além disso, vários estudos relataram resultados favoráveis do uso de gabapentina e memantina em pacientes com síndrome do nistagmo infantil.
CONCLUSÕES: Embora existam tratamentos ópticos, farmacológicos e cirúrgicos para síndrome do nistagmo infantil, mais pesquisas são necessárias para ampliar os tipos de tratamentos, pois o tratamento não cura a doença de forma definitiva e completa.
Palavras-chave: Nistagmo congênito; Distúrbios da motilidade ocular; Doenças oculares.
INTRODUCTION
Infantile nystagmus syndrome (INS) is an oculomotor disorder of unknown etiology, clinically characterized by involuntary rhythmic oscillations of the eyes, in which they move back and forth constantly. INS may arise at birth or in the first years of life1-3. The occurrence in the general population is about 0.14%4. The eyes tend to oscillate on the horizontal axis, although vertical and/or twisting movements may be secondary. Since nystagmus is bilateral and usually conjugated, the amplitude of the movement is similar in both eyes (OU), ranging from 0.3º–15.7º, with an average frequency of 2–3 Hz5,6. In addition, INS can occur spontaneously or be hereditary (X-linked, recessive or dominant)7, and mutations have also been found in the FRMD gene7,8.
In 1967, Cogan et al. published an article dividing this syndrome into sensory nystagmus and motor nystagmus, depending on the type of eye movement (pendular and saccadic or jerk, respectively); however, in 1974, he clarified that the type of movement would not be enough for this division, since in some gaze positions, a nystagmus classified as sensory, for example, could have a saccadic component, thus making this classification obsolete9.
In INS, the eyes oscillate constantly and predominantly on the horizontal axis, although twisting or vertical movement may be present as a secondary component. Another manifested symptom is head tilt, a vicious position that does not depend on eye sway and does not seem to be linked to any associated condition.5 Visual acuity (VA) is variable, depending on the presence of some associated eye condition5,10,11. The diagnosis is predominantly clinical, but complementary tests can be useful to clarify the etiology, such as optical coherence tomography (OCT)12-15 and electroretinography (ERG)16-21.
Given the relevance of this issue and its impact on the population’s vision, the objective of the present work was to conduct a literature review on INS, addressing diagnostic methods, optical, pharmacological, and surgical treatments.
METHODS
A literature review was conducted by searching PubMed and LILACS databases covering the years 2000 to 2022. The following terms were searched: “Nystagmus,” “Infantile nystagmus,” “Nystagmus diagnosis,” “Nystagmus treatment,” “Electroretinography,” and “Optical coherence tomography.” These terms were searched to address INS in general, its diagnosis, including complementary tests and its management. Exclusion criteria included articles published before 2000, duplicate articles, case reports not published in PubMed or LILACS, and articles that were not related to the review topic.
PubMed and LILACS databases were used because they are reliable indexing sources for searching scientific articles and have many scientific publications. A meta-analysis was not applicable in this manuscript due to the heterogeneity of the articles used in this review, containing animal and human studies, and is therefore a qualitative rather than quantitative analysis of the data.
RESULTS
Taking into account the search conducted on PubMed and LILACS, applying the filters and exclusion criteria, 57 articles were selected for the present review, as detailed in figure 1.
DISCUSSION
1. Diagnosis
1.1. Clinical presentation
Nystagmus can be detected and identified without the aid of eye movement recordings, with the clinical observation of rhythmic and repetitive oscillations of the eyes, with or without sensory deficits6,22. INS is one of several types of nystagmus that are usually seen in the early months of life, with ocular oscillation, well-described clinical features, but not diagnostic, being important in determining prognosis23.
Recording eye movement allows you to determine the waveform of the nystagmus motion, which is described in terms of amplitude, frequency, and overall pattern or shape of oscillation24,25. In addition, the intensity of the nystagmus is calculated by multiplying its amplitude by its frequency, which represents the average speed of eye movements26. It is important to note that the intensity of nystagmus in INS decreases with eyelid closure and convergence and disappears with sleep6.
It is worth noting that in most cases, the eyes oscillate constantly and predominantly on the horizontal axis (uniplanar movement). However, torsional or vertical motion may be present as a secondary component, with the presence of conjugated movements that increase in intensity with eccentric gaze and increased fixation effort5,6.
The waveforms can be jerk, pendular, or a combination of both. Jerk-type waveforms are characterized by accelerating slow movements away from the fixture, interspersed with the resumption of active phase “jumps” that bring fovea back to the object of consideration. The pendular waveforms are dominated by slow, smooth eye movements toward and away from fixation. Importantly, children who remain in the pendular waveform for longer time tend to have greater vision impairment than those who progress from this waveform to the jerk waveform.23 It should be noted that there are 12 waveforms of congenital nystagmus ranging from purely pendular to jerk5,25.
Unlike patients with acquired nystagmus, patients with INS rarely present with the complaint of oscilloscopy, sometimes reporting the symptom intermittently, triggered, for example, by fatigue, stress, visual effort, or even excitement5,22,27-29. Patients may present with head sway, or head tilting, a vicious position that does not depend on eye sway and does not seem to be linked to any associated ocular condition, and is not compensatory, based on the INS described mainly in its mature form in older children and adults5,6,24.
VA is variable, depending on the presence of some associated eye conditions, ranging from 0.2 to 0.5 logMAR, especially in cases of nystagmus in albinism, which manifests a VA of approximately 0.6 to 0.9 logMAR5,10,11.
Among the associated ocular disorders, the most frequent are ametropias, such as hyperopia, myopia, and corneal astigmatism30-32.
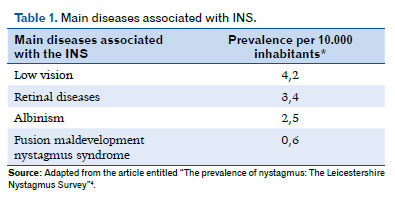
Furthermore, although most patients with INS have only idiopathic nystagmus, there are still about 40% who have associated comorbidities, such as albinism, retinal dystrophies, aniridia, optic nerve disorders, achromatopsia, and congenital cataracts (Table 1)4,24,33.
1.2. Complementary diagnostic tests
Although the diagnosis of INS is predominantly clinical, caution is needed to determine its etiology. A complete ophthalmologic examination is essential, noting the presence and nystagmus characteristics and performing biomicroscopy to analyze iris transillumination and retina. In addition, it should include the evaluation of head posture, VA at different viewing angles, and cycloplegic refraction, which are critical for effective therapeutic intervention24.
Complementary examinations, such as OCT12-15 and ERG, allow specific causes to be determined16-21. In addition, gene sequencing, either conventional Sanger sequencing (FRMD7 and GPR143) or next-generation gene sequencing, can aid in etiologic estimation34.
OCT is a noninvasive imaging method able to detect changes in retina and fovea12. For infants and young children, a probe has been developed to perform OCT in a portable manner that can be performed quickly, making it an essential tool for identifying retinal morphology in retinopathy of prematurity, maculopathy, retinal dystrophy, post-traumatic choroidal neovascularization, and Leber’s congenital amaurosis (LCA)13-15.
ERG is a minimally invasive technique that provides a direct and objective assessment of retinal function.16 It can be used as an auxiliary tool in the diagnosis of nystagmus associated with retinal diseases such as LCA, cone dystrophy, achromatopsia, and congenital stationary night blindness (CSNB)19, as well as spasmus nutans, a rare idiopathic acquired disorder of childhood that comprises a clinical triad: asymmetric pendular nystagmus, torticollis, and upward and downward head tilt17-18. ERG results tend to be normal in patients with infantile nystagmus of idiopathic etiology; however, those with a mutation in FRMD7 may have ERG consistent with foveal hypoplasia, and albinos may manifest an alteration of ganglion cell numbers19-21.
The sensitivity of ERG and visual evoked potential (VEP) have not been extensively studied for the classification of congenital nystagmus; however, VEP demonstrates misrouting of fibers in ocular albinism, optic nerve hypoplasia, and chiasmatic glioma19.
It is possible to perform other tests such as magnetic resonance imaging, visual fields, and angiofluoresceinography motivated by clinical findings that indicate their performance, whether it is a positive family history for a disorder associated with nystagmus, iris transillumination, optic nerve abnormality, abnormal retinal pigmentation, or systemic neurological signs35.
Importantly, cortical areas play an important role in controlling saccadic and pursuit functions. With the involvement of the brainstem, vestibular nuclei, cerebellum, and visual cortex in patients with nystagmus, infratentorial lesions are expected in up to 40% of patients with multiple sclerosis36. Magnetic resonance imaging (MRI) of the brain is useful for nutans spasmus type nystagmus because it has been associated with diencephalic and optic nerve tumors. MRI is also the imaging method of choice for investigating patients with descending nystagmus, with approximately a quarter of cases being due to a potentially treatable abnormality35,37.
1.3. Genetic study
There is evidence for seven NYS gene loci: Xq26.2 [NYS1 (MIM 31 0700)], 6p12 [NYS2 (MIM)], 7p11.2 [NYS3 (MIM 60 8345)], 13q31-q33 [NYS4 (MIM 19 3003)], Xp11.4 [NYS5 (ME 30 0589)], Xp22.3 [NYS6 (ME 30 0814)], and 1q31-q32.238. The most frequent cause would be X-linked, due to mutation of the FRMD7 gene, located in the NYS1 locus (Xq26.2)20,39, in addition to autosomal dominant and autosomal recessive inheritance7.
2. Treatment
2.1. Optical
Initially, ametropia correction should be performed because more than half of the children with INS have refractive errors, such as astigmatism, which can manifest itself in the first 10 years40-42. Both the prevalence and magnitude of astigmatism increase in the first 8 years of children with INS32.
In patients, in whom nystagmus is reduced when the eyes converge, prisms can be used to induce convergence. Proper convergence can be produced by placing a pair of prism 7 diopter (7Δ), time-based prisms with a lens addition of one less spherical diopter (−1.00 sph) to compensate for induced accommodation. If the patient responds to the prisms, he will also respond to the retraction of both medial rectus muscles43.
The benefit of using optical prisms has been observed in patients with binocular vision; however, those with INS who have alternate fixation (i.e., those who have ocular deviation and no binocular vision) will not benefit from this optical aid44.
Optical treatment is sometimes sufficient to correct the anomalous head position. Some authors, such as Hertle, advocated the prescription of complete cycloplegic correction and anisometropia in patients with nystagmus and in those under 10 years of age, and Reinecke, who agreed with the use of complete correction of hyperopia in children with nystagmus45.
2.2. Pharmacological
Studies have reported good results with the use of memantine and gabapentin for treating INS46-48.
Memantine can be started at low doses, such as 10 mg daily for several days, and dosage can be gradually increased (for example, 20 mg daily for several days, 30mg daily for several days, and then 40mg daily). Adverse effects are usually mild and tolerable (for example, headache, fatigue, and dizziness). As for gabapentin, higher doses may be required to achieve a beneficial response (e.g., up to 2,400 mg per day); adverse effects are also usually mild and tolerable, such as dizziness, ataxia, and fatigue44.
Acetazolamide can be effective at a dosage of 250 to 1000 mg twice a day; however, this drug has many adverse effects that are barely tolerable, including paresthesia, lethargy, kidney stones, and tinnitus, as well as the rare but serious adverse effects of agranulocytosis and aplastic anemia44. One study concluded that drug treatment of nystagmus with topical brinzolamide was effective in a sample of 23 patients, but further studies are still needed33. Another study analyzed the effect of baclofen in eight patients, and it was concluded that this drug may be effective in a select group of patients with congenital periodic alternating nystagmus, a type of INS. Thus, it might be feasible to attempt treatment before considering surgical intervention in this condition49.
Treatment with botulinum toxin, an important neurotoxin used in various medical procedures, has been used successfully in some cases of nystagmus associated with strabismus50.
2.3. Surgical
Surgical treatment of INS has been performed for decades, with indications to correct the anomalous head position and/or improve VA and is offered to minimize nystagmus with convergence in patients with associated strabismus51-53. They are usually performed when the child is between 7 and 8 years old54. In this review, the Anderson–kestenbaum procedure, the Cüppers divergence procedure, tenotomy procedure, and muscle reinsertion are discussed. However, it is important to stress that despite the variety of surgical interventions available, there are no clear consensus and accepted clinical guidelines on relative efficacy and safety, and there are not enough data in the literature to determine whether some procedures are better than others for INS55.
In the early 1950s, Anderson and Kestenbaum independently published their hypotheses on the attenuation of anomalous head position in INS. Anderson defended the idea that there was a need for retrogression of the agonist rectus muscles, whose action would be toward the vicious position of the head. In the case of blockage in the right gaze, the lateral rectus of the abducting eye and the medial rectus of the abducting eye would be retracted. Kestenbaum advocated resection and retraction of the four horizontal rectus muscles, 4-10mm, with the intention of increasing image permanence in the fovea45,53,54. Thus, this procedure would involve shifting from the null position (a single position in which nystagmus would be minimal and VA would be improved) to the primary position, by the combined movement of the extraocular muscles, thus relieving the anomalous position of the head53. Initially, these surgeries were done only for correcting torticollis. After this correction, patients with large refractive errors would be offered the additional benefit of being able to direct their gaze through the optical centers of their glasses and thus gain an improvement in their VA45.
In the following decades, the techniques were improved by researchers such as Parks, Calhoun, Harley, and Nelson, among others, and their variations were used to correct the anomalous position of the head, with the correction of an eccentric blocking point in patients with INS, enlargement of the blocking zone, and reduction of the intensity of nystagmus outside this zone52-54. Parks modified Kestenbaum’s technique, performing the 5-6-7-8 technique (totaling 13 mm in each eye), in which a 7-mm retraction is performed on the lateral rectus muscle, a 6-mm resection of the medial rectus of the abducting eye, a 5-mm retraction of the other medial rectus, and finally an 8-mm resection of the lateral rectus of the abducting eye. However, this technique presented high rates of recurrence of the anomalous head position over time, as did Calhoun and Harley’s procedures. Nelson’s procedure aimed to reduce the need for further surgery in the future by performing a 40% increase in resection (7; 8.4; 9.8; and 11.2 mm) for head rotations up to 30° and a 60% increase (8; 9.6; 11.2; 12.8 mm) for head rotations of 45° or more53,54.
In the early 1970s, Cüppers introduced an artificial divergence procedure that placed the patient’s eyes in a position that constantly converged and therefore decreased the amplitude and severity of nystagmus in the primary eye position, resulting in improved VA44,52,53. This procedure consisted of retracting the medial rectus muscles to diverge the eyes in patients with convergence-suppressed nystagmus53. Consequently, a convergence effort would be required for a wider view, and nystagmus would be suppressed44. However, for the Cüppers operation to be successful, the patient should show improvement of nystagmus during convergence, in addition to having binocular fusion and stereopsis criteria that few nystagmus sufferers have. If the Cüppers procedure was not properly evaluated preoperatively, there could be hypercorrection and exotropia postoperatively, requiring further surgery53.
The four-muscle tenotomy procedure would involve isolating each horizontal rectus muscle (two medial and two lateral rectus) with an absorbable polyglycolic acid suture placed on each muscle immediately posterior to its insertions, then moving on to disinsertion of each muscle and immediate reinsertion at the same site as the insertions. Initially, such a procedure was performed in animals, with favorable results supporting the hypothesis that tenotomy and reinsertion of the horizontal extrinsic eye muscles could improve nystagmus in humans. The tenotomy and reinsertion procedure could be used to enlarge the blocking zone of INS and reduce the intensity of nystagmus44. In 2003, the first test of four-muscle tenotomy was published in 10 adults with INS and no previous history of eye surgery. Nine patients showed significant improvement in nystagmus and subjective visual functions with this surgical procedure alone1,53. In 2004, a study was published with five children with INS, also with no previous history of surgery, contributing to the idea that tenotomy of all four muscles would be effective for treating INS; however, because the jerk waveforms did not show changes, it was hypothesized that this technique was more resilient for treating INS2,53,56. In addition to these researches, more studies have shown positive results56,57.
In conclusion, it was possible to perform a qualitative analysis, showing that the diagnosis of INS would be mainly clinical; however, complementary exams could provide guidance, for example, on the prognosis and how the treatment could be directed. Also, it was possible to observe that although there are optical, pharmacological, and surgical treatments for INS, more research is still needed to broaden the therapeutic options because treatment would not always be able to completely cure the nystagmus.
REFERENCES
1. Hertle RW, Dell’Osso LF, FitzGibbon EJ, Thompson D, Yang D, Mellow SD. Horizontal rectus tenotomy in patients with congenital nystagmus: results in 10 adults. Ophthalmology. 2003;110(11):2097-105.
2. Hertle RW, Dell’Osso LF, FitzGibbon EJ, Yang D, Mellow SD. Horizontal rectus muscle tenotomy in children with infantile nystagmus syndrome: a pilot study. J AAPOS. 2004;8(6):539-48.
3. Nystagmus in Infancy and Childhood [Internet]. American Academy of Ophthalmology. [Internet]. 2018 [cited 2022 Aug 15]. Available from: https://www.aao.org/disease-review/nystagmusin-infancy-childhood
4. Sarvananthan N, Surendran M, Roberts EO, Jain S, Thomas S, Shah N, et al. The prevalence of nystagmus: The Leicestershire Nystagmus Survey. Invest Ophthalmol Vis Sci. 2009;50(11):5201-6.
5. Abadi RV, Bjerre A. Motor and sensory characteristics of infantile nystagmus. Br J Ophthalmol. 2002;86(10):1152-60.
6. Hertle RW, Maldanado VK, Maybodi M, Yang D. Clinical and ocular motor analysis of the infantile nystagmus syndrome in the first 6 months of life. Br J Ophthalmol. 2002;86(6):670-5.
7. Jia X, Zhu X, Li Q, Jia X, Li S, Guo X. Novel mutations of FRMD7 in chinese patients with congenital motor nystagmus. Mol Med Rep. 2017;16(2):1753-8.
8. Tarpey P, Thomas S, Sarvananthan N, Mallya U, Lisgo S, Talbot CJ, et al. Mutations in FRMD7, a newly identified member of the FERM family, cause X-linked idiopathic congenital nystagmus. Nat Genet. 2006;38(11):1242-4.
9. Dell’Osso LF, Hertle RW, Daroff RB. “Sensory” and “motor” nystagmus: erroneous and misleading terminology based on misinterpretation of David Cogan’s observations. Arch Ophthalmol. 2007;125(11):1559-61.
10.Weiss AH, Kelly JP. Acuity development in infantile nystagmus. Invest Ophthalmol Vis Sci. 2007;48(9):4093-9.
11. Wildsoet CF, Oswald PJ, Clark S. Albinism: its implications for refractive development. Invest Ophthalmol Vis Sci. 2000;41(1):1-7.
12. Pilat A, Sibley D, McLean RJ, Proudlock FA, Gottlob I. High-Resolution Imaging of the Optic Nerve and Retina in Optic Nerve Hypoplasia. Ophthalmology. 2015;122(7):1330-9.
13. Lee H, Proudlock FA, Gottlob I. Pediatric Optical Coherence Tomography in Clinical Practice-Recent Progress. Invest Ophthalmol Vis Sci. 2016;57(9):OCT69-79.
14. Lee H, Sheth V, Bibi M, Maconachie G, Patel A, McLean RJ, et al. Potential of handheld optical coherence tomography to determine cause of infantile nystagmus in children by using foveal morphology. Ophthalmology. 2013;120(12):2714-24.
15. Lee H, Proudlock F, Gottlob I. Is handheld optical coherence tomography reliable in infants and young children with and without nystagmus? Invest Ophthalmol Vis Sci. 2013;54(13):8152-9.
16. Wilsey LJ, Fortune B. Electroretinography in glaucoma diagnosis. Curr Opin Ophthalmol. 2016;27(2):118-24.
17. Kiblinger GD, Wallace BS, Hines M, Siatkowski RM. Spasmus nutans-like nystagmus is often associated with underlying ocular, intracranial, or systemic abnormalities. J Neuroophthalmol. 2007;27(2):118-22.
18. Smith DE, Fitzgerald K, Stass-Isern M, Cibis GW. Electroretinography is necessary for spasmus nutans diagnosis. Pediatr Neurol. 2000;23(1):33-6.
19. Brecelj J, Stirn-Kranjc B. Visual electrophysiological screening in diagnosing infants with congenital nystagmus. Clin Neurophysiol. 2004;115(2):461-70.
20. Thomas MG, Crosier M, Lindsay S, Kumar A, Araki M, Leroy BP, et al. Abnormal retinal development associated with FRMD7 mutations. Hum Mol Genet. 2014;23(15):4086-93.
21. Mcketton L, Kelly KR, Schneider KA. Abnormal lateral geniculate nucleus and optic chiasm in human albinism. J Comp Neurol. 2014;522(11):2680-7.
22. Lee AG, Brazis PW. Localizing forms of nystagmus: symptoms, diagnosis, and treatment. Curr Neurol Neurosci Rep. 2006; 6(5):414-20.
23. Felius J, Muhanna ZA. Visual deprivation and foveation characteristics both underlie visual acuity deficits in idiopathic infantile nystagmus. Invest Ophthalmol Vis Sci. 2013;54(5):3520-5.
24. Khanna S, Dell’Osso LF. The diagnosis and treatment of infantile nystagmus syndrome (INS). ScientificWorldJournal. 2006 Oct 30;6:1385-97.
25. Penix K, Swanson MW, DeCarlo DK. Nystagmus in pediatric patients: interventions and patient-focused perspectives. Clin Ophthalmol. 2015 Aug 21;9:1527-36.
26. Hanson KS, Bedell HE, White JM, Ukwade MT. Distance and near visual acuity in infantile nystagmus. Optom Vis Sci. 2006;83(11):823-9.
27. Salehi Fadardi M, Abel LA. Saccades under Mental Load in Infantile Nystagmus Syndrome and Controls. Optom Vis Sci. 2018;95(4):373-83.
28. Salehi Fadardi M, Bathke AC, Harrar SW, Abel LA. Task-induced Changes in Idiopathic Infantile Nystagmus Vary with Gaze. Optom Vis Sci. 2017;94(5):606-15.
29. Jones PH, Harris CM, Woodhouse JM, Margrain TH, Ennis FA, Erichsen JT. Stress and visual function in infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2013;54(13):7943-51.
30. Healey N, McLoone E, Mahon G, Jackson AJ, Saunders KJ, McClelland JF. Investigating the relationship between foveal morphology and refractive error in a population with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2013;54(4):2934-9.
31. Sampath V, Bedell HE. Distribution of refractive errors in albinos and persons with idiopathic congenital nystagmus. Optom Vis Sci. 2002;79(5):292-9.
32. Wang J, Wyatt LM, Felius J, Stager DR Jr, Stager DR Sr, Birch EE, et al. Onset and progression of with-the-rule astigmatism in children with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):594-601.
33. Aygit ED, Ocak OB, İnal A, Fazıl K, Akar S, Gokyigit B. The effects of topical carbonic anhydrase inhibitor in treatment of nystagmus. Int Ophthalmol. 2018;38(1):265-9.
34. Rim JH, Lee ST, Gee HY, Lee BJ, Choi JR, Park HW, et al. Accuracy of Next-Generation Sequencing for Molecular Diagnosis in Patients with Infantile Nystagmus Syndrome. JAMA Ophthalmol. 2017;135(12):1376-85.
35. Bertsch M, Floyd M, Kehoe T, Pfeifer W, Drack A V. The clinical evaluation of infantile nystagmus: What to do first and why. Ophthalmic Genet. 2017;38(1):22-33.
36. Iyer PM, Fagan AJ, Meaney JF, Colgan NC, Meredith SD, Driscoll DO, et al. Horizontal nystagmus and multiple sclerosis using 3-Tesla magnetic resonance imaging. Ir J Med Sci. 2016; 185(4):881-6.
37. Bronstein AM, Miller DH, Rudge P, Kendall BE. Down beating nystagmus: magnetic resonance imaging and neuro-otological findings. J Neurol Sci. 1987;81(2-3):173-84.
38. Radhakrishna U, Ratnamala U, Deutsch S, Bartoloni L, Kuracha MR, Singh R, et al. Novel homozygous, heterozygous and hemizygous FRMD7 gene mutations segregated in the same consanguineous family with congenital X-linked nystagmus. Eur J Hum Genet. 2012;20(10):1032-6.
39. Thomas MG, Crosier M, Lindsay S, Kumar A, Thomas S, Araki M, et al. The clinical and molecular genetic features of idiopathic infantile periodic alternating nystagmus. Brain. 2011;134(3):892-902.
40. Noval S, González-Manrique M, Rodríguez-Del Valle JM, Rodríguez-Sánchez JM. Abnormal head position in infantile nystagmus syndrome. ISRN Ophthalmol. 2012;2011:594848.
41. Fresina M, Benedetti C, Marinelli F, Versura P, Campos EC. Astigmatism in patients with idiopathic congenital nystagmus. Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1635-9.
42. Jayaramachandran P, Proudlock FA, Odedra N, Gottlob I, McLean RJ. A randomized controlled trial comparing soft contact lens and rigid gas-permeable lens wearing in infantile nystagmus. Ophthalmology. 2014;121(9):1827-36.
43. Hertle RW. Examination and refractive management of patients with nystagmus. Surv Ophthalmol. 2000;45(3):215-22.
44. Thurtell MJ, Leigh RJ. Treatment of nystagmus. Curr Treat Options Neurol. 2012;14(1):60-72.
45. Ospina LH. Dealing with Nystagmus. J Binocul Vis Ocul Motil. 2018;68(4):99-109.
46. Shery T, Proudlock FA, Sarvananthan N, McLean RJ, Gottlob I. The effects of gabapentin and memantine in acquired and congenital nystagmus: a retrospective study. Br J Ophthalmol. 2006;90(7):839-43.
47. McLean R, Proudlock F, Thomas S, Degg C, Gottlob I. Congenital nystagmus: randomized, controlled, double-masked trial of memantine/gabapentin. Ann Neurol. 2007;61(2):130-8.
48. Bögli SY, Afthinos M, Huang MY. Effect of Gabapentin/Memantine on the Infantile Nystagmus Syndrome in the Zebrafish Model: Implications for the Therapy of Ocular Motor Diseases. Invest Ophthalmol Vis Sci. 2017;58(7):3149-57.
49. Comer RM, Dawson EL, Lee JP. Baclofen for patients with congenital periodic alternating nystagmus. Strabismus. 2006; 14(4):205-9.
50. Sabetti L, Guetti F, Pomanti V, Murano G. Botulinum Toxin for the Treatment of Nystagmus Associated with Exotropia. Open J Ophthalmol. 2022;12(4):392-6.
51. Gräf M. Kestenbaum and artificial divergence surgery for abnormal head turn secondary to nystagmus. Specific and nonspecific effects of artificial divergence. Strabismus. 2002; 10(2):69-74.
52. Köse S, Egrilmez DG, Uretmen O, Celebisoy N, Pamukçu K. Retroequatorial recession of horizontal recti with loop suture in the treatment of congenital nystagmus. Strabismus. 2003;11(2):119-28.
53. Greven MA, Nelson LB. Four-muscle tenotomy surgery for nystagmus. Curr Opin Ophthalmol. 2014;25(5):400-5.
54. Lee J. Surgical management of nystagmus. J R Soc Med. 2002; 95(5): 238-41.
55. Cham KM, Abel LA, Busija L, Kowal L, Bachar Zipori A, Downie LE. Surgical interventions for infantile nystagmus syndrome. Cochrane Database Syst Rev. 2021;2021(2):CD013390.
56. Wang Z, Dell’Osso LF, Jacobs JB, Burnstine RA, Tomsak RL. Effects of tenotomy on patients with infantile nystagmus syndrome: foveation improvement over a broadened visual field. J AAPOS. 2006;10(6):552-60.
57. Wang ZI, Dell’Osso LF. Tenotomy procedure alleviates the “slow to see” phenomenon in infantile nystagmus syndrome: model prediction and patient data. Vision Res. 2008;48(12):1409-19.
AUTHORS INFORMATION




Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
June 4, 2022.
Accepted on:
May 6, 2023.