Julia Alves Utyama; Guilherme Novoa Colombo Barboza; Roberta de Júlio Matheus; Marcello Novoa Colombo Barboza
DOI: 10.17545/eOftalmo/2021.0024
ABSTRACT
The herpes simplex virus (HSV) is a common pathogen that is characterized by its high worldwide prevalence. HSV1 can cause ocular conditions and is spread through infected saliva or direct contact with active lesions. Primary infection can be asymptomatic and self-limited, leading to virus latency in sensory ganglia, such as the trigeminal ganglion. Reactivation of the infection usually results from immunocompromising factors, febrile infections, trauma, or exposure to sunlight, and its severity is directly related to the host’s immune capacity. Another relevant aspect is the increasing incidence of measles in Brazil and worldwide in recent years. Measles is a highly contagious viral disease that manifests with rashes, high fever, runny nose, and conjunctivitis. Against this background, it is essential to understand that immunological changes caused by the virus may be the main determinant of the course of the disease and its complications, of which otitis media, pneumonia and keratoconjunctivitis are the most common. The objectives of this study were to describe a case of multifocal herpetic keratitis concomitant with active measles infection in a young, immunocompetent patient and to emphasize the importance of awareness of HSV infection and its opportunistic nature.
Keywords: Herpetic keratitis; Herpes simplex; Measles; Conjunctivitis.
RESUMO
O Vírus Herpes Simples (HSV) é um patógeno comum, caracterizado por sua alta prevalência mundial. O HSV-1 é o responsável pelos quadros oculares e sua transmissão ocorre através da saliva infectada ou do contato direto com lesões ativas. A infecção primária pode ser assintomática e autolimitada, levando à latência do vírus nos gânglios sensoriais, como o gânglio trigêmeo. A reativação da infecção normalmente decorre de fatores imunocomprometedores, infecções febris, traumas ou exposição solar e sua severidade está diretamente relacionada à capacidade imunológica do hospedeiro. Outro aspecto relevante é o número crescente de casos de sarampo no Brasil e no mundo nos últimos anos. Trata-se de uma doença viral altamente contagiosa, que se manifesta com exantemas, febre alta, coriza e conjuntivite. E frente à este cenário, é fundamental entender que as alterações imunológicas provocadas pelo vírus podem ser o principal fator determinante do curso da doença e suas complicações, onde otite média, pneumonia e ceratoconjuntivite são as mais comuns. O objetivo deste estudo é relatar um caso de ceratite herpética multifocal concomitante à infecção ativa por sarampo em paciente jovem e imunocompetente, além de ressaltar a importância do pronto conhecimento da infecção pelo HSV e sua característica oportunista.
Palavras-chave: Ceratite herpética; Herpes Simples; Sarampo; Conjuntivite.
INTRODUCTION
The herpes simplex virus (HSV) is a common human pathogen, classified into HSV1 and HSV2. HSV1 is characterized by extragenital conditions and spreads through infected saliva or active lesions, whereas HSV2 involves perigenital sexually transmitted diseases1. In addition, it is endemic in virtually all human societies2. A study has shown that 18.2% of cadavers aged 0-20 years have HSV in the trigeminal ganglion, as detected by a molecular polymerase chain reaction (PCR) test, reaching almost 100% of cadavers over a period of 60 years3.
Herpetic eye infection may be primary or recurrent. Primary HSV infection may present as acute or asymptomatic, self-limited keratoconjunctivitis. After first contact, the virus remains latent in the sensory ganglia until viral replication is reactivated. In most cases, the recurrence of infection is caused by triggering factors such as febrile infections, exposure to sunlight, and physical or emotional exhaustion, and it can affect anterior structures such as the eyelids, conjunctiva, cornea, and iris as well as the posterior segment of the eyeball4,5.
Protection against the virus predominantly depends on T cells, which gives the virus an opportunistic nature. Therefore, in addition to the virulence of the virus and vulnerability of the affected tissue, the individual’s immune defense mechanism can determine the severity and presentation of the infection6,7.
In recent years, cases of measles have been reported in various parts of the world. According to the country’s Ministry of Health, Brazil had outbreaks in all of its five regions, with 15,734 reported cases and 7,939 confirmed cases from December 2019 to September 2020, with the highest incidence in the states of Pará, São Paulo, Paraná, and Santa Catarina8. Measles is an exanthematic, infectious, and highly contagious disease caused by an RNA virus of the Paramyxoviridae family that is transmitted from 4 to 6 days before the exanthema appears until 4 days after it. The clinical picture is characterized by high fever, generalized maculopapular exanthema, Koplik’s spots in the oropharynx, runny nose, and conjunctivitis9. The clinical picture of measles in the eyes usually presents a papillary-follicular conjunctival reaction with hyperemia, which may or may not compromise the cornea and evolve with complications such as keratitis, ocular perforation, and chorioretinitis10,11. Additionally, studies show that measles infection can cause abnormalities in the immune response of monocytes and lymphocytes, which causes temporary immunosuppression in affected individuals and increases the risk of secondary infections during that period12-14.
In view of the current increase in the incidence of measles in Brazil and the high prevalence of HSV in the population, understanding the immunosuppressive nature of measles and the opportunistic nature of HSV becomes essential for diagnosis and treatment. Therefore, this study aims to describe an atypical ocular condition of herpetic keratitis in an immunocompetent young patient with active measles infection.
CASE REPORT
A 24-year-old man visited the outpatient service of Visão Laser Eye Hospital in the city of Santos, State of São Paulo, Brazil, with a complaint of redness and foreign body sensation in the right eye (OD) for 8 days, accompanied by cough and runny nose. The patient also reported that 4 days after the onset of symptoms, he developed high fever and reddish rashes that started in the retroauricular region, progressed throughout the body, and were still present at the time of the consultation. With the appearance of the lesions, he sought to be admitted to the emergency room, where he underwent a serology test that was IgM-positive for measles. He denied comorbidities and any relevant ophthalmologic history. A general physical examination revealed a red maculopapular skin rash on the limbs, trunk, and face, with desquamative areas. On ophthalmologic examination, his uncorrected visual acuity (VA) was found to be 20/20 in both eyes (OU). Biomicroscopy revealed conjunctival hyperemia 2+ with follicular reaction in the tarsal conjunctiva and mucous secretion in OD; left eye (OS) without changes. Lubricating eye drops one drop four times a day and cold compresses three times a day were prescribed, and the patient was asked to return after 7 days.
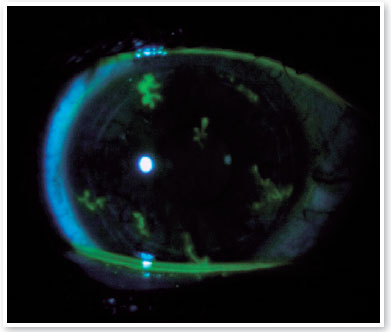
Three days after the first evaluation, the patient reported a decrease in VA in OD and photophobia. On examination, the right cornea showed fluorescein-positive dendritic lesions on the entire corneal surface, characterizing diffusely distributed herpetic keratitis (Figure 1). Acyclovir 400 mg orally five times a day (2 g per day) for 10 days was prescribed. The lubricating eye drops were maintained at four times a day, and the patient was asked to return for re-evaluation after 5 days. Upon returning, the patient had no complaints, and OD showed a significant decrease in the size and number of herpetic lesions, which resolved completely after 10 days of antiviral treatment.
DISCUSSION
HSV is a globally endemic pathogen, and its only known reservoir is the human species. Studies that tested for the virus in the trigeminal ganglion using PCR reported that HSV is present in at least 90% of the world population up to 60 years of age in its latent form15-17. Herpetic keratitis is the most common ophthalmic condition in recurrent infection, and its pathophysiology is directly related to viral replication in the corneal epithelium. However, distinct clinical manifestations may occur, depending on the affected corneal layer and the pathophysiological mechanism involved4. Infectious epithelial keratitis usually presents with characteristic localized vesicles and dendritic ulcers. However, in immunodeficient individuals who are unable to contain the viral replication, clinical manifestations may be more evident. The literature suggests that any condition that depresses the cellular-mediated immune response, such as post-transplantation treatment18,19, diabetes mellitus20, measles21,22, or HIV infection23, are predisposing factors for atypical, more severe, and recurrent conditions of herpetic keratitis4,24,25.
Measles is an acute and highly contagious viral disease, and humans are the only reservoir of the virus. In addition to the characteristically high fever, generalized vasculitis is the most significant factor responsible for the characteristic clinical manifestations of measles, but its morbidity and mortality can be more often attributed to increased susceptibility to secondary infections9,26. Many studies have shown that the immune response to the measles virus is paradoxically associated with its suppression against other antigens, and this may persist for weeks after the resolution of the clinical picture24,26,27. Abnormalities in both the innate immune response of monocytes and the adaptive lymphocyte response have been reported after infection with the measles virus28,29.
Although there are data in the literature that support a relationship between measles virus infection and HSV, few studies have focused on clarifying the pathophysiological mechanisms that may link these diseases. Therefore, as exemplified by the case herein reported and considering the high incidence of measles and the high prevalence of HSV in the population, it is concluded that it is essential to recognize herpetic keratitis as a possible complication of measles. In addition, due to the immunosuppressive state of patients with measles, it is important to consider possible variations in clinical presentation, as adequate follow-up and treatment are essential for the preservation of vision and of the ocular structures.
REFERENCES
1. Tagliari NAB, kelmann RG, Diefenthaler H. Therapeutic aspects of infections causedby Herpes Simplex Virus Type-1. Perspectiva, Erechim. Março/2012. v.36, n.133, p.191-201.
2. Umene K, Sakaoka H. Evolution of herpes simplex virus type 1 under herpesviral evolutionary processes. Arch Virol. 1999; 144(4):637-56.
3. Liedtke W, Opalka B, Zimmermann CW, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci. 1993;116(1):6-11.
4. Freitas D, Alvarenga L, Lima ALH. Ceratite herpética. Sociedade brasileira de lente de contato e córnea. Arq Bras Oftalmol. 2001;64(1):81-6.
5. Pavan-Lagston D. Diagnosis and Manegement of Herpes Simplex Ocular Infection. Int Ophthalmol Clin. 1975;15(4):19-35.
6. Whittle HC, Smith JS, Kogbe OI, Dossetor J, Duggan M. Severe ulcerative herpes of mouth and eye following measles. Trans R Soc Trop Med Hyg. 1979;73(1):66-9.
7. White ML, Chodosh J. Herpes Simplex Virus Keratittis: A treatment guideline. Department Of Ophthalmology Harvard Medical School. June/2014.
8. Ministério da Sáude, Secretaria de Vigilância em Saúde. Informe semanal Sarampo no Brasil - semanas epidemiológicas 1 a 37, 2020. Informe Nº39. Vol 51, Out 2020. Disponível em: https://antigo.saude.gov.br/images/pdf/2020/October/07/Boletim-epidemiologico-SVS-39--1-.pdf
9. Ministério da Saúde, Guia de vigilância epidemiológica. Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Caderno 2. Sarampo. Brasília 2009; 7ª edição.
10. Kayikçioglu Ö, Kir E, Söyler M, Güler C, Irkeç M. Ocular findings in a measles epidemic among young adults. Ocul Immunol Inflamm. 2000;8(1):59-62.
11. Sandford-Smith JH, Whittle HC. Corneal ulceration following measles in Nigerian children. Br J Ophthalmol. 1979;63(11):720-4.
12. Beckford AI, Kaschula ROC, Stephen C. Factors associated with fatal cases of measles: a retrospective autopsy study. S Afr Med J. 1985:68(12):858-63.
13. Miller DL. Frequency of complications of measles, 1963. Report on a national inquiry by the public health laboratory service in collaboration with the society of medical officers of health. Br Med J. 1964:2(5401):75-8.
14. Morley D. Severe measles in the tropics. I. Br Med J. 1969;1(5639):297-300.
15. Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der Keyl H, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74(24):11464-71.
16. Hill J, Ball M, Neumann D, Azcuy AM, Bhattacharjee PS, Bouhanik S, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82(16):8230-4.
17. Bustos D, Atherton S. Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest Ophthalmol. Vis Sci. 2002;43(7):2244-9.
18. Papanicolau GA, Meyers BR, Fuchs WS, Guillory SL, Mendelson MH, Sheiner P, et al. Infectious ocular complications in orthotopic liver transplant patients. Clin Infect Dis. 1997;24)6):1172-7.
19. Pfefferman R, Gombos G, Kountz S. Ocular complications after renal transplantation. Ann Ophthalmol. 1977;9(4):467-70, 473.
20. Geerlings S, Hoepelman A. Immune dysfunction in patients in patients with Diabetes Mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-65.
21. Ukety TO, Martens K. Ocular ulcerative herpes following measles in Kinshasa, Zaire. Curr Eye Res. 1991;10 Suppl:131-7.
22. Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppress cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186(6):813-23.
23. Hodge WG, Margolis T. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology. 1997;104(1):120-4.
24. Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16(4):375-86.
25. Bennet J, Dolin R, Blaser M. Principles and practice of infectious disease. Vol I. 7a ed. 2009, pp 1642, 1682, 1713. Elsevier.
26. Griffin DE, Ward BJ, Esolen LM. Pathogenesis of Measles Virus Infection: An Hypothesis for Altered Immune Responses. J Infect Dis. 1994;170 Suppl 1:S24-31.
27. Moss WJ, Griffin DE. Measles. Lancet. 2012;379(9811):153-64.
28. Griffin DE. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176-89.
29. Avota E, Gassert E, Schneider-Schaulies S. Measles virus-induced immunosuppression: from effectors to mechanisms. Med Microbiol Immunol. 2010;199(3):227-37.
AUTHOR’S INFORMATION
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
July 21, 2020.
Accepted on:
December 15, 2020.