Annelise Lídice Francisquini Fernandes Marra1; Cássio Augusto Macanhão2; Fernando dos Reis Spada3; Astor Grumann Júnior4
DOI: 10.17545/eOftalmo/2021.0022
ABSTRACT
PURPOSEE: This study has as objective to analyze the association of different formulas on intraocular lenses calculation with the refractive result on cataract surgery
METHODS: Retrospective cohort study including 200 eyes from 200 patients submitted to phacoemulsification with the insertion of the spherical intraocular lens (IOL). The data was arranged on Windows Excel software and exported to the Statistical Package for the Social Sciences (SPSS) program. The Student T test was used with the same level of significance (p<0.05) in all analyses.
RESULTS: The 165 patients classified as surgical success were the ones with final refraction of 0.00D (±0.5D), of whom 140 (83.33%) used the formula SRKT. It was yet considered biometric success those patients who obtained ±0.5D refraction on the spherical in relation to the expected refraction by its biometry, this way 49.5% reached biometric success. From these: 46.4% achieved biometric success with Hoffer Q, 48.8% with SRKT, and 50% with Haigis.
CONCLUSION: The SRKT and Hoffer Q formulas have showed good precision for surgical success and regular precision to evaluate biometric success on eyes with average axial length. There was a better result on the biometric success of myopic patients on the preoperative than the hyper myopic ones.
Keywords: Cataract; Biometry; Phacoemulsification; Refractive Errors.
RESUMO
OBJETIVOS: O objetivo deste estudo foi analisar a associação das diferentes fórmulas para o cálculo da lente intraocular com o resultado refrativo na cirurgia de catarata.
MÉTODOS: Coorte histórica incluindo 200 olhos de 200 pacientes submetidos à facoemulsificação com colocação da lente intraocular (LIO) esférica. Os dados foram tabulados no software Windows Excel e exportados para o programa Statistical Package for the Social Sciences (SPSS). Foi utilizado o teste T de Student e o mesmo nível de significância (p<0,05) em todas as análises.
RESULTADOS: Foram classificados como sucesso cirúrgico os 165 pacientes com refração final de 0,00D (±0,5D), sendo que 140 (83,33%) deles usaram a fórmula SRKT. Foi, ainda, considerado sucesso biométrico aqueles pacientes que obtiveram refração ±0,5D no esférico em relação à refração esperada pela sua biometria, deste modo 49,5% atingiram o sucesso biométrico. Destes: 46.4% tiveram sucesso biométrico com Hoffer Q, 48,8% com SRKT, e 50% com Haigis.
CONCLUSÃO: A fórmula SRKT e Hoffer Q demonstraram uma boa precisão para o sucesso cirúrgico e uma precisão mediana para calcular o sucesso biométrico em olhos de comprimento axial médio. Houve um melhor resultado para o sucesso biométrico nos pacientes míopes no pré-operatório do que nos hipermetropes.
Palavras-chave: Catarata; Biometria; Facoemulsificação; Erros de refração.
INTRODUCTION
Human vision results from the integrated functioning of the retina, pupil, cornea, iris, optic nerve, and crystalline lens subsystems. Images are obtained by focusing light on the retina through the lens, which produces images through the optic nerve(1). For light transmission to occur with clarity, the lens must be transparent, which depends on correctly shaped cells and on a compact arrangement of their proteins(2). Thus, cataracts can be defined as opacity of the lens(3). When this occurs, images lose their sharpness, and visual acuity is reduced(4).
According to global statistical data, the main risk factor for cataract formation is aging(5). In addition, there are other possible causes, such as eye trauma, smoking, ultraviolet radiation, diabetes, and congenital cataracts(6).
Phacoemulsification is currently the most widely used surgical technique in most developed countries due to the possibility of rapid visual recovery and to its low rate of pre- and postoperative complications(7). Phacoemulsification is performed by making a small incision in the cornea, through which the opacified lens is fragmented, aspirated(8), and finally replaced with an artificial intraocular lens (IOL)(9).
Patients who undergo phacoemulsification expect better vision, and phacoemulsification minimizes the postoperative refractive error, which may result in the elimination of the need for glasses(10). This requires the IOL to be accurately calculated and properly implanted(11).
To achieve greater success in the IOL implantation procedure, it is essential to obtain the axial length (AL), keratometry, and depth measurements of the anterior chamber(12). These measurements can be obtained using an ultrasonic biometer, which emits sounds and generates reflected echoes propagated through the eyeball structures(13).
In this respect, a device called an optical biometer uses a light beam without contact with the patient to obtain the required measurements to calculate the AL. Similar to optical coherence tomography, optical biometers use the partial coherence interferometry (PCI) technique(13).
According to Monteiro et al.(13), when using the PCI method, the AL is determined by two coaxial light beams that focus on the anterior surface of the cornea and on the pigmented epithelium of the retina, eliminating the influence of longitudinal eye movements. North American and European studies have stated that 95% of cataract cases can be examined using optical biometry(13).
Biometric precision, which is associated with improved surgical techniques and the evolution of IOLs, can correct pre-existing ametropia, in addition to the patient’s cataract. Therefore, optical biometry is the most important ophthalmologic examination to calculate the IOL and is capable of accurately estimating the patient’s final sphere component. Thus, the phakectomy procedure has a high success rate and a low complication rate(12).
Thus, the need to accurately calculate the dioptric power of the IOL arises(14). Several formulas have been proposed to determine the optimal choice of lens to be implanted. First- and second-generation formulas (SRK and SRK II) showed better results in eyes with average AL (22–24.50 mm). Third-generation formulas led to significant improvements in eyes with an AL of >24.50 mm (SRK T) and <22 mm (Hoffer Q and Holladay)(15). Fourth-generation formulas (Holladay 2 and Haigis) demonstrated having solved problems arising from different dimensions of the eyeball, except in cases of extreme myopia(16,17). Finally, fifth-generation formulas were developed, namely, Olsen and Barrett Universal II. The latter is called “Universal” because it performs well when calculating IOLs for eyes with all possible AL(18). These IOL calculations are based on the measurement of the anterior chamber depth (ACD), AL, lens thickness, and keratometry (K)(13).
As mentioned above, calculating IOLs for cataract surgery is still a challenge. Therefore, to obtain significantly successful postoperative refractive results, it is essential to validate and evaluate new formulas to calculate IOL power(19). With this background, the present study aimed to evaluate different formulas for calculating IOL and the refractive result in cataract surgery.
METHODS
This historical cohort study evaluated 200 eyes of 200 patients who underwent phacoemulsification with aspheric IOL placement (model SN60WF) from January 2012 to December 2016.
Preoperative and postoperative visual acuity were assessed from the best visual correction, using the Snellen scale, and for statistical analysis, the data were transformed to the LogMAR scale. Postoperative refraction results were evaluated at least 3 months after phacoemulsification.
Patients of both sexes aged ≥18 years who were diagnosed with cataract and underwent phacoemulsification with aspheric IOL implantation were included. Patients who had previously undergone refractive surgery in either eye and those who underwent phacoemulsification surgery in the contralateral eye during the study period were excluded. Thus, only one eye was considered in this study, even for those for whom this technique was performed bilaterally. The sample was selected sequentially rather than randomly during the study period.
IOL was calculated using AL, central keratometry values (K1 and K2), and the expected dioptric power of the IOL, measured with non-contact PCI (IOL Master, version 3.01, Carl Zeiss Meditec, Jena, Germany). IOLs were calculated according to the different ALs using the Hoffer Q, SRK T, and Haigis formulas. For comparative analysis, the Holladay 1 formula was also used, in addition to the formulas already described. Then, the values obtained with each formula were compared with the refractive result in cataract surgery. The dependent variable used was the formula to calculate IOL (Holladay 1, Hoffer Q, SRK T, or Haigis). The independent variables were demographic characteristics, namely, sex, age, and the eye operated, and clinical characteristics, namely, history of previous ophthalmic surgery, AL (16 to 32 mm), and initial and final refraction (0 to ±10 diopters).
All phacoemulsifications in this study were performed with a microincision in the cornea to remove the opacified lens and, subsequently, to insert the IOL. Alcon’s Centurion® phacoemulsifier was used for this purpose.
For analysis, surgical and biometric successes were considered in the present study. Patients who achieved a plano refraction or a variation of up to ±0.50 D in the sphere component in the final postoperative evaluation after cataract surgery were considered surgical successes. Biometric success was determined from the expected spherical refraction in the biometric calculations performed by IOL Master and compared with the patient’s final refraction. Patients who had at most a ±0.50 D deviation from the refraction expected from the biometric calculation were classified as biometric successes.
Qualitative data were described as frequencies (simple and relative) and were analyzed using the chi-squared test. Quantitative data were demonstrated by measures of central tendency (mean) and their respective measures of variability/dispersion (amplitude [maximum and minimum] and standard deviation). The mean value calculated with the formulas was presented for each group of independent variables, and the significance of the differences in the means were calculated using Student’s t-test and analysis of variance. The pre-established significance level was 95% confidence interval (p≤0.05).
The data were collected according to the inclusion criteria, after approval by the Research Ethics Committee of the University of South Santa Catarina (CEP-UNISUL), under Certificate of Presentation for Ethical Appraisal No. 80422817.0.0000.5369. The information was accessed from electronic medical records after the password was made available by their guardian.
RESULTS
A total of 200 medical records of patients who underwent phacoemulsification surgery with IOL implantation from 2012 to 2016 were evaluated. The mean age of the patients undergoing surgery was 71.7 (±8.07 SD) years (35-90 years), with 106 (53%) right eyes and 94 (47%) left eyes. Regarding the sex of the patients, 128 (64%) were women and 72 (36%) were men.
Preoperative and postoperative visual acuity, assessed with the best correction on the LogMAR scale, ranged from 0.00 to 1.3 (mean 0.29, ± 0.23 SD) preoperatively and from 0.00 to 1.00 (mean 0.05, ± 0.05 SD) postoperatively. The spherical refraction ranged from −5.00 to +5.75 D preoperatively, with a mean of +1.27D (±1.82 SD), and from −2.50 to +1.50 postoperatively, with a final mean of -0.45D (±0.48 SD), as shown in table 1.
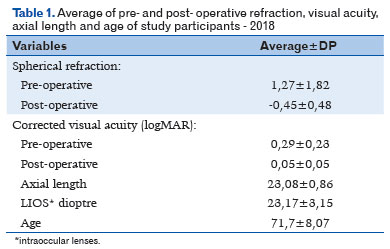
AL ranged from 21.14 mm to 25.38 mm, with a mean of 23.08 mm (± 0.86 SD). For the diopters of the IOLs used in the surgeries, the mean was 23.17D (±3.15 SD), ranging from 16.50 D to 35.00 D.
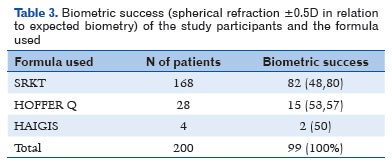
The SRK T, Hoffer Q, and Haigis formulas were used for biometric calculation of the IOL in the preoperative period. The SRK T formula was used in 168 (84%) patients, Hoffer Q in 28 (14%), and Haigis in 4 (2%).
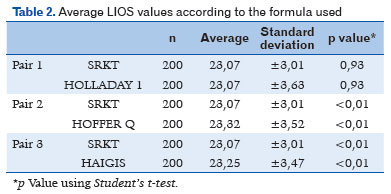
Following transposition of the actual values of the biometric calculations obtained preoperatively for the 200 patients to the other formulas, the mean IOL values were 23.07 D (±3.01 SD) for SRK T, 23.32 D (±3.52 SD) for Hoffer Q, 23.25 D (± 3.47 SD) for Haigis, and 23.07 D (± 3.47 SD) for Holladay. A statistically significant correlation was found between the formulas and the mean IOL diopters (p<0.01), except between SRK T and Holladay (p=0.93), as shown in table 2.
Surgical success, defined as explained above, occurred in 165 (82.5%) of the patients, while 35 (17.5%) did not achieve it.
Of the 128 women, 106 (82.6%) achieved surgical success, and of the 72 men in the study, 59 (81.9%) achieved surgical success, with no statistically significant difference between males and females (p=0.877).
Of the 165 patients who achieved surgical success, the right eye was operated in 91 (55.2%) and the left eye in 74 (44.8%), with no statistically significant correlation between the operated eye and surgical success (p=0.186).
Regarding the use of formulas and surgical success, of 168 patients for whom the SRK T formula was used, surgery was successful in 140 (83.3%) patients. The mean IOL power was 22.84 D (±2.88 SD). If the Hoffer Q formula had been used in these 168 patients, the mean IOLs would have been 23.09 D (± 3.35 SD); for Haigis, it would have been 23.05 D (± 3.32 SD), and for Holladay 22.98 D (± 3.1 SD). The difference in the mean IOL power was statistically significant between formulas (p<0.01).
Biometric success was achieved in 99 (49.5%) patients, whereas 101 (50.5%) did not achieve it (Table 3).
Of the 128 women, 65 (50.8%) achieved biometric success and 63 (49.2%) did not. Among the 72 men, 34 (47.22%) achieved biometric success and 38 (52.8%) did not, with no statistically significant difference between males and females (p=0.629).
Biometric success was achieved in operated right eyes in 54 (50.94%) patients and in left eyes in 45 (47.87%) patients, with no statistically significant correlation between the operated eye and the success of the biometric calculation (p=0.665).
The mean keratometry values in patients who achieved biometric success were K1 = 43.48 (±1.82 SD) and K2 = 44.49 (±1.71 SD), whereas in those who did not achieve biometric success, these values were K1 = 43.44 (±1.78 SD) and K2 = 44.28 (±1.82 SD), with no statistically significant correlation between the keratometry values (K1 and K2) and biometric success (p=0.175).
In the 166 patients who had been hyperopic preoperatively, the mean postoperative spherical refraction was -0.07 D (±0.23 SD), with biometric success achieved in 77 (46.38%) patients. In the 34 patients with preoperative myopia, the mean postoperative spherical refraction was -0.26D (±0.19 SD), with biometric success achieved in 21 (61.7%) patients.
The 99 patients with biometric success had a mean final spherical refraction of -0.18 D, and the 101 patients who did not achieve biometric success had a mean spherical refraction of -0.71 D, with a mean refractive difference between successful and unsuccessful cases of -0.53 D, which was not statistically significant (p=0.267).
Regarding the use of formulas and biometric success, of the 168 patients for whom the SRK T formula was used, 82 (48.80%) achieved biometric success and 86 (51.20%) did not (Table 3).
The mean IOL diopter in biometrically successful patients for whom SRK T was used was 22.92 D (±2.86 SD). If these calculations had been performed using Hoffer Q, the mean diopter would have been 23.18 (± 3.33 SD), with 23.14 (±3.33 SD) for Haigis and 23.07 (±3.10 SD) for Holladay. The difference in the mean IOLs between the formulas was statistically significant (p<0.01).
Of the 28 patients for whom the Hoffer Q formula was used, 15 (53.57%) achieved biometric success and 13 (46.43%) did not, as shown in table 3. The mean IOL dioptric power for these 28 patients was 24.50 D (±4.19 SD).I If the SRK T formula had been used, the mean IOL dioptric power would have been 24.28 D (± 3.52), with 24.21 D (± 3.52 SD) for Haigis and 24.33 D (±3.83) for Holladay. The difference in the mean IOL diopters was statistically significant between the formulas (p<0.01).
The mean IOL dioptric power in patients who had biometric success with the Hoffer Q formula (15 patients) was 24.13 D (±3.52 SD). If the SRK T formula had been used, the mean IOL dioptric power would have been 23.93 D (±4.30 SD), with 23.93 D (±5.10 SD) for Haigis and 24.06 (4.73 SD), for Holladay. The difference in the mean IOL diopters was statistically significant between the formulas (p<0.01).
The Haigis formula was used in 2% of the sample (four patients), half of whom (two patients) achieved biometric success, as shown in table 3.
DISCUSSION
In this study, no significant correlation was found between surgical and biometric success and sociodemographic factors such as age and sex. This was demonstrated in the studies by Hayashi et al.(20) and Reitblat et al.(21), who also found no statistical correlation between these variables.
The present study found a substantial improvement in the final visual acuity of the patients after the surgical procedure, resulting in plano final refraction ± 0.5 D in the sphere component (considered surgical success) and a mean postoperative visual acuity on the LogMAR scale of 0.05 (± 0.05 SD). This was also demonstrated in the study by Chiacchio et al.(22), who found a mean preoperative visual acuity of 0.64 LogMAR and 0.09 LogMAR postoperatively.
Aristodemou et al.(23) concluded that in the AL range from 21.5 to 26 mm, there was no statistically significant difference between the formulas used for biometric calculation, which is in line with the findings of the present study, which used the SRK T, Hoffer Q, and Haigis formulas in patients within that AL range. It is important to note that the study conducted by Shajari et al.(24), in which nine formulas for calculating IOL values were analyzed, concluded that the greatest accuracy was obtained using the Barrett and Olsen formulas, which are fifth-generation formulas. In accordance with the present study, Shajari et al. determined that the use of different formulas (SRK T, Haigis, or Hoffer Q) resulted in different IOL values.
Regarding biometric success, in the present study 49.5% of the patients achieved it. Thus, it can be stated that approximately half of the patients studied had a refraction difference of ±0.5 D in relation to the refraction estimated by the biometric calculation. When comparing these results with the literature, Rodrigues et al.(12) observed that 55% of the individuals achieved results of ±0.5 D.
In the study by Monteiro et al.(13), 40.7% of the cases studied had a residual refraction of up to ±0.50 D using the SRK T formula. This value is close to that found in the present study, which obtained 48.8% biometric success (residual refraction of ±0.5 D) with the SRK T formula. In a prospective study of 33 eyes submitted to phacoemulsification with IOL implantation using the SRK T formula, with the AL ranging from 22.5 to 24.5 mm, Lagrasta et al.(25) found a refractive error of ±0.50 D in 55% of the patients, with the AL and biometric success rate similar to those in the present study.
In a study by Corrêa et al.(14), 81 patients undergoing phacoemulsification surgery and IOL implantation were analyzed using the SRK T formula, with similar epidemiological data to those of the present study, such as a mean age of 62.5 years, and biometric success was achieved with a residual refraction of ±0.5 D in 40.7% of the patients, a figure close to that found in the present study.
The study by Rodrigues et al.(12) obtained a biometric success of ±0.50 D in 55% of the right eyes and 46% of the left eyes when using the Haigis formula, a percentage close to that found in the present study for that formula. Since the sample of patients for whom the Haigis formula was used was extremely small in the present study, it was not possible to compare its results accurately with the literature data. Camps et al.(26) used the Haigis formula for calculation in patients who had previously undergone refractive keratotomy, reaching a plano spherical equivalent in 40% of the patients and up to ±1 in 80% of the patients studied.
One explanation for the difference between surgical and biometric success is the surgeon’s experience in considering the tendency to hyperopia of third-generation formulas. Thus, it is often decided to seek a final IOL refraction slightly slanted toward myopia in order to achieve emmetropia. This can be observed when comparing the mean preoperative spherical refraction of +1.27 D (±1.82 SD) with the postoperative mean of -0.45 D (±0.48 SD). It must be noted that a mean refractive difference of approximately 0.5 D was observed in the present study between patients who achieved biometric success and those who did not.
According to Rodrigues et al.(12), when selecting the formula for myopic patients, the Haigis and SRK T formulas increased the accuracy of the refractive result, indicating that these formulas are more precise than Holladay 1. In the study by Cetinkaya et al.(27), in which patients were highly myopic (AL 26-33mm), the SRK T formula was used and resulted in ±1D postoperative refraction in 51.2% of the patients studied. Also according to Cetinkaya et al., phacoemulsification allowed 51.6% of patients with cataracts and high myopia to achieve a postoperative refraction within the expected target, unlike the present study, in which biometric success was achieved in 61.7% of myopic patients.
Regarding hyperopic patients, the study by Akaishi et al.(28) showed a statistically significant improvement in the mean postoperative visual acuity in hyperopic patients. By contrast, in the present study hyperopic patients achieved a mean spherical refraction of -0.07D (±0.23 SD) postoperatively, and only 77 (46.38%) of those patients achieved biometric success. Thus, it can be stated that patients who were myopic in the preoperative period had a higher tendency to achieve biometric success than patients who were hyperopic in the preoperative period.
In the present study, it was observed that for most patients, third-generation formulas (SRK T and Hoffer Q) were used, and a fourth-generation formula (Haigis) was used for only 2% of the patients. Although the results obtained are similar to those found in the literature - that is, a final refractional result of plano ±0.5D in approximately 50% of the patients -, there is still uncertainty regarding the effects of the proper use of a given formula on the final surgical and biometric success. In view of the above, in order to improve the performance of the formulas for biometric calculation, it is recommended that the ocular characteristics of each patient be considered and analyzed.
The SRK T and Hoffer Q formulas demonstrated good accuracy for surgical success and moderate accuracy for calculating biometric success in eyes with average AL using IOL Master, version 3.01. The fourth-generation Haigis formula did not have a large enough quantitative sample in this study. The results for biometric success were better for patients who were myopic in the preoperative period than for those who were hyperopic preoperatively. It should also be noted that the surgeon’s experience was crucial for the patient’s final surgical success. Thus, it can be concluded that the choice of the formula used in biometric calculation remains a challenging decision, which must take into account numerous variables and consider each patient’s individual characteristics to obtain the best results.
REFERENCES
1. Acharya RU, Yu W, Zhu K, Nayak J, Lim T-C, Chan JY. Identification of Cataract and Post-cataract Surgery Optical Images Using Artificial Intelligence Techniques. J Med Syst. 2010;34(4):619-28.
2. Figueirêdo SE. Estudo de alterações estruturais nos genes CRYAA, CRYGC E CRYGD em pacientes com catarata congênita de uma população brasileira [tese de doutorado]. Campinas: Universidade Estadual de Campinas; 2013.
3. Sheeladevi S, Lawrenson JG, Fielder AR, Suttle CM. Global prevalence of childhood cataract: a systematic review. Eye. 2016;30(9):1160-9.
4. Grałek M, Kanigowska K, Seroczyńska M. Cataract in children-not only an ophthalmological problem. Medycyna Wieku Rozwojowego. 2007;11(2):227-30.
5. Taleb A, Ávila M, Moreira H. As condições de saúde ocular no Brasil - 2009. 1ed. São Paulo: Conselho Brasileiro de Oftalmologia; 2009.
6. Yi J, Yun J, Li Z-K, Xu C-T, Pan B-R. Epidemiology and molecular genetics of congenital cataracts. Int J Ophthalmol. 2011;4(4):422-32.
7. Nieves-Moreno M, Asorey-García A, Santos-Bueso E, García-Sánchez J. Historia de la cirugía de cataratas (I): desde el abatimiento hasta la extracción. Arch Soc Esp Oftalmol. 2015;e1-3.
8. Desai P, Reidy A, Minassian DC. Profile of patients presenting for cataract surgery in the UK: national data collection. Br J Ophthalmol. 1999;83(8):893-6.
9. Grumann Junior A, Flügel N, Ritta P. An analysis of the visual results when using toric lenses in cataract surgery. Rev Bras Oftalmol. 2015;74(1):12-5.
10. Rubenstein JB, Raciti M. Approaches to corneal astigmatism in cataract surgery. Curr Opin Ophthalmol. 2013;24(1):30-4.
11. Rezende F, Bisol RR, Bisol T. Troca do cristalino com finalidade refrativa (TCR). Rev Bras Oftalmol. 2009;68(3):180-7.
12. Rodrigues WF, Vidal CLL, Mendonça RLA, Silva ER. Biometria ocular, estimativa matemática e variação esférica pós-facectomia. Rev Bras Oftalmol. 2015;74(6):350-4.
13. Monteiro EL, Allemann N. Biometria óptica. Arq Bras Oftalmol. 2001;64(4):367-70.
14. Corrêa ZM da S, Kronbauer FL, Goldhardt R, Marcon ÍM, Bakowicz F. Precisão ecobiométrica da fórmula SRK/T na facoemulsificação. Arq Bras Oftalmol. 2001;64(3):233-7.
15. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16(3):333-40.
16. Zaldivar R, Shultz MC, Davidorf JM, Holladay JT. Intraocular lens power calculations in patients with extreme myopia. J Cataract Refract Surg. 2000;26(5):668-74.
17. Hoffer KJ. Clinical results using the Holladay 2 intraocular lens power formula. J Cataract Refract Surg. 2000;26(8):1233-7.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of Intraocular Lens Calculation Formulas. Am Acad Ophthalmol. 2018;125(2):169-78.
19. Feijóo B, Ferreira T, Zabala L, Guerra P, Gonçalves C, Couceiro J, et al. Comparação de metodologias actuais para cálclo da potência da lente intra-ocular. Revista Sociedade Portuguesa de Oftalmologia. 2017;41(4):9-16.
20. Hayashi K, Ogawa S, Yoshida M, Yoshimura K. Influence of Patient Age on Intraocular Lens Power Prediction Error. Am J Ophthalmol. 2016;(170):232-37.
21. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: Comparison of methodologies. J Cataract Refract Surg. 2016; 42(2):217-25.
22. Chiacchio BB, Sato RM, Siqueira RB, Marques FF. Fidelidade do “potential acuity meter” (PAM) no prognóstico da acuidade visual pós-operatória de cirurgia de catarata. Arq Bras Oftalmol. 2008;71(6):805-8.
23. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011; 37(1):63-71.
24. Shajari M, Kolb CM, Petermann K, Böhm M, Herzog M, de’Lorenzo N, et al. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J Cataract Refract Surg. 2018;44(8):942-48.
25. Lagrasta JM. Clinical results in phacoemulsification using the SRK/T formula. Arq Bras Oftalmol. 2009;72(2):189-93.
26. Camps VJ, Lorens DPP, Fez D, Coloma P, Caballero MT, Garcia C, et al. Algorithm for Correcting the Keratometric Estimation Error in Normal Eyes. Optom Vis Sci. 2012;89(2):221-8.
27. Cetinkaya S, Acir NO, Cetinkaya YF, Dadaci Z, Yener HI, Saglam F. Phacoemulsification in eyes with cataract and high myopia. Arq Bras Oftalmol. 2015;78(5):286-9.
28. Akaishi L, Tzelikis PF. Primary piggyback implantation using the ReSTOR intraocular lens: case series. J Cataract Refract Surg. 2007;33(5):791-5
AUTHOR’S INFORMATION



Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
June 16, 2020.
Accepted on:
January 28, 2021.