Leonardo José Barbosa1; Ahlys Ayumi Nagai Miyazaki1,2; Marianna Almeida Hollaender1; Júlia Furtado Heringer1; Stefanie Hitomi de Nishi Lee1; Ana Vega Carreiro de Freitas1; Amaryllis Avakian Shinzato1; Pedro Carlos Carricondo1
DOI: 10.17545/eOftalmo/2021.0017
ABSTRACT
Cataract, the opacification of the crystalline lens, is the leading cause of blindness in the world according to the World Health Organization. In the 21st century, cataracts represent 51% of the causes of blindness in the population worldwide. Although the study of the physiology of vision was initiated more than 3000 years ago and the first written reports of cataract surgery date back to the 1st century, a solution has not yet been found for universal access to opacified lens removal surgery. Cataract reversibility with lens removal surgery (phacectomy) makes it necessary for the medical community to seek methods to improve access to this treatment, and understanding the history of cataract surgery can help in this quest. New surgical techniques continue to emerge, and training programs aimed at increasing the number of ophthalmologists who are able to diagnose and perform opacified lens extraction surgery can benefit patients without access to treatment. History shows that even more than two millennia after the start of cataract surgeries, equipment, materials, and surgical techniques are still constantly evolving. The history of cataract is described in this article.
Keywords: Cataract; Surgery; History.
RESUMO
A catarata, opacificação do cristalino, é a principal causa de cegueira no mundo segundo a Organização Mundial de Saúde (OMS). Em pleno século XXI, a catarata representa 51% das causas de cegueira da população mundial. Apesar de a compreensão da fisiologia da visão ter iniciado há mais de 3000 anos e os primeiros relatos escritos de cirurgia de catarata datarem do século I, ainda não se encontrou uma solução mundial para o acesso universal à cirurgia de remoção do cristalino opacificado. A reversibilidade da catarata com a cirurgia de remoção do cristalino (facectomia), torna necessário que a comunidade médica busque métodos para difundir o acesso a esse tratamento e o entendimento da história da cirurgia de catarata pode ajudar nessa busca. Novas técnicas cirúrgicas continuam surgindo e a capacitação para o aumento no número de médicos oftalmologistas capazes de diagnosticar e realizar a cirurgia de extração do cristalino opacificado pode beneficiar pacientes sem acesso ao tratamento. A história evidencia que, mesmo após mais de dois milênios do início da realização do procedimento de catarata, equipamentos, materiais e técnica cirúrgica permanecem em constante evolução. A história da catarata é descrita nesse artigo.
Palavras-chave: Catarata; Cirurgia; História.
INTRODUCTION
Even in the 21st century, cataract is still the cause of 51% of the cases of blindness diagnosed in the world population1. Although the physiology of vision has been studied for over 3000 years and the first written reports of cataract surgery date back to the 1st century2,3, a worldwide solution for universal access to opacified lens removal surgery has not yet been found. Unlike irreversible diseases such as glaucoma and age-related macular degeneration, the reversibility of cataract with lens removal surgery (phacectomy) makes it necessary for the medical community to seek methods to improve access to this treatment, and understanding the history of cataract can help in this quest. New surgical techniques continue to emerge, and programs for training opthalmologists in diagnosing and performing phacectomy can ultimately lead to an increase in the number of ophthalmologists qualified to perform this surgery; this may also benefit patients without access to treatment.
Studies on the anatomy and physiology of the eye were initiated more than 3000 years ago2,3. Unfortunately, many of the old records have been lost. The oldest of them include the descriptions made by Hippocrates (400 BC) and Aristotle (350 BC), who described the human eye in detail, albeit with some misconceptions, such as believing that the crystalline lens corresponded with a postmortem accumulation of substances.
Any opacification of the lens that can cause the degradation of its optical quality, causing symptoms and visual loss, is called a cataract. Although the first anatomical descriptions of the lens and its zonules date back to the 1st century and were reported by Celsus, Rufus, and Galen, the optical function of the lens was established only in the 16th century by Franciscus Manrolycus. Until that time, cataracts were believed to be similar to glaucoma, as both led to blindness and “whitening of the eye.” Only in 1650 did Rolfinck describe cataracts as an opacification of the lens. This notion was initially rejected by the vast majority of physicians and was only accepted a century later4.
The first record of cataract surgery dates back to the 5th century BC, describing the dislocation of the lens to the vitreous cavity through the application of a blunt force directly to the eyeball with a rounded instrument; the application of this force caused the detachment or rupture of the zonular fibers and subsequently the displacement of the lens and its capsule due to the destruction of their supporting structures9 (Figure 2). Unfortunately, this technique is still used in developing countries, such as Nigeria and other West African countries10.

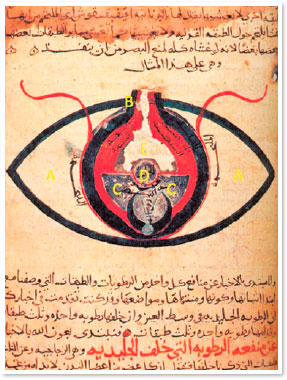
Centuries later, Avicenna modified this technique and started using a sharp, pointed instrument, which was supposed to be inserted into the eye, to break the zonular fibers and cause lens displacement5. According to Avicenna, surgery should not be performed in places with a lot of light. During the procedure, the patient was supposed to look at the nose, while the surgeon inserted a pointed instrument (meghdahah) through the limbus until it reached the anterior chamber and was able to mobilize the lens (Figure 1,3). The cataract was then pushed to the lower angle of the anterior chamber until the pupillary axis was released. Therefore, the opacified lens was not removed from the patient’s eye5.

The first surgical removal of the lens was performed in Paris in 1748. In 1752, Jacques Daviel presented his work titled “A new method for curing cataracts by extracting the lens.”6 The technique involved the extraction of the entire opaque crystalline lens through a wide corneal incision and opening the anterior capsule. It was essential that the lens remained intact while performing the extraction. Thus, the procedure was reserved for “mature” lenses so hardened that they would not break during the extraction procedure; this limited this surgery to only very advanced cataracts. As sutures was not available at that time, patients remained immobilized with sandbags around their heads until complete healing occurred; this type of immobilization led to the risk of death from pulmonary embolism. The surgical technique was called extracapsular extraction of the crystalline lens; it was used for some time, then abandoned, and revived just two centuries later.
At the same time, other techniques emerged, such as intracapsular extraction, which was initially described by St. Yves in 1722. It was perfected by Samuel Sharp followed by George Beer in 17994.
This technique gained even more acceptance from the 19th century after the advent of general anesthesia techniques and the presentation of the topical anesthetic effect of cocaine by Karl Köller. Use of local anesthesia in ophthalmology was presented at the Heidelberg Congress on September 15, 1884, and was immediately accepted worldwide7,8.
Except for the extraction of the diseased lens through a broad corneal incision and the innovations in anesthesia, not much changed in terms of the surgical technique until the end of the 19th century. Intracapsular extraction began to gain popularity because of the studies by MacNamara, Molrony, and Smith in India12. As there was no surgical microscope, cortex removal using extracapsular extraction was difficult; it resulted in intense inflammation and rapid opacification of the posterior capsule. In contrast, removal of the entire lens with the capsular sac via the intracapsular technique did not result in these complications. After World War II, due to the improvement of intracapsular extraction with iridectomy, some physicians believed that this surgical technique could not be further improved13.
However, after the introduction of the surgical microscope in 1950 by Harms and Barraquer, the emergence of more innovations, such as the use of an enzyme to dissolve the zonules (Joaquín Barraquer, 1958) or cryoextraction (T. Krawczyk, 1961), facilitated the intracapsular removal of the lens, improving surgical results14. Even after surgery, patients remained aphakic, requiring heavy glasses with positive lenses popularly known as “bottle bottoms” for refractive correction.
In 1949, Englishman Harold Ridley developed the first artificial intraocular lens. As a military surgeon during World War II, Ridley had observed that under certain conditions, glass and acrylic seemed to be inert with regard to tissues. Shattered fragments that penetrated the eyes of aircraft crew seemed to generate insignificant tissue reaction, except if pointed parts were in contact with a moving portion of the eye15. On November 29, 1949, Ridley implanted the first intraocular lens, marking an important change in ophthalmology (Figure 4). Interestingly, the material he used at that time, polymethylmethacrylate, is still used in many currently implanted intraocular lenses15. However, the first implanted lenses were large, heavy, and positioned in the anterior segment of the eye, generating a large number of complications. After about 10 years, Cornelius Birkhorst developed a lens better suited to the human eye from the Ridley model. In addition to enhancing Ridley’s intraocular lens, Birkhorst also determined that the best surgical technique for his implant would be extracapsular, because it preserves the posterior capsule, allowing an optimal positioning of the lens in the posterior segment of the eye16.

The greatest innovation of the 20th century occurred with the introduction of phacoemulsification by Dr. Charles Kelman in 196717 (Figure 5). In 1965, he suggested that the ultrasound device used by dentists could be adapted for fragmentation of the crystalline lens into small pieces that could then be aspirated. After two years of hard work, Kelman introduced his ingenious phacoemulsification equipment18. By this method, through an opening in the anterior capsule, the lens would be fragmented by ultrasound and aspirated through the equipment’s own cannula. In this way, the surgeon would work in a closed system, avoiding large incisions, and consequently, excessive exposure of the interior of the eyeball19. The first phacoemulsification in a human eye took 76 minutes, and the equipment was large, inefficient, difficult to control, and extremely heavy18. Initially, this technique was greatly criticized as it involved costs for equipment acquisition and maintenance, team training, and a learning curve for the surgeon, and it did not seem to offer superior surgical outcomes when compared with the techniques most commonly used at the time20.

Extracapsular surgery remained the standard procedure until the development of Healon (Pharmacia Corp.; currently produced by Advanced Medical Optics, Inc., Santa Ana, CA, USA), a viscoelastic substance, by Robert Stegmann and David Miller in the mid-1980s. This innovation dramatically increased the popularity of phacoemulsification by allowing a significant decrease in endothelial damage, stimulating the development of new similar substances20. With the increase in the number of supporters of this new surgical technique that involved a small incision, the development of foldable intraocular lenses that could be implanted through smaller and smaller incisions was also initiated21. Currently, there are lenses that can be implanted through incisions as small as 1.8 mm22. In the search for the best surgical result and the implant closest to the anatomy of a healthy crystalline lens, the continuous circular capsulorhexis technique was created by Gimbel in 199023. Thus, the possibility of intraocular lens implantation in the capsular bag became safer and more predictable24, allowing the surgeons to focus on the refractive aspect of cataract surgery, i.e., trying to obtain the lowest residual refraction after the phacoemulsification procedure by using lens implants with a refractive power as close as possible to the value required for focusing the images on the retina.
The predictability of the procedure made it possible for the development of intraocular lenses that could compensate not only for the loss of spherical refractive power because of the removal of the lens (spherical intraocular lenses) but also for other optical aberrations, such as asphericity (aspheric intraocular lenses)25 and corneal toricity (toric intraocular lenses)26. The surgical removal of the crystalline lens drastically suppresses lens accommodation, and patients undergoing implantation of these types of intraocular lenses remain dependent on glasses for close vision and reading. In order to be independence from optical correction, the industry continuously offers new models of intraocular lenses that perform refractive correction for both far and near vision. There are bifocal (far and near vision correction) and trifocal (far, near, and intermediate vision correction) lenses available, and more recently, extended focus lenses, which provide constantly clear vision at all distances (far-intermediate or intermediate-near)27.
The recent emergence of new technologies demonstrates a growing intention to facilitate learning and continues to make cataract surgery safer and more predictable. These new technologies include the use of the femtosecond laser (femtosecond laser-assisted cataract surgery) for performing certain steps, such as incisions, capsulotomy, and nucleus fracture, in cataract surgery, 28.
Although technology has advanced rapidly over the past 30 years, more than half the patients diagnosed with blindness worldwide have cataracts as their primary eye disease1. Training new ophthalmologists to diagnose such a prevalent eye disease and perform cataract removal surgery, whether extracapsular or by phacoemulsification, makes professionals available to the general population with the necessary preparation to meet the needs of patients from all socioeconomic and cultural levels.
The training of resident physicians remains the backbone of specialization in Brazil. Most hospitals with appropriate training programs belong to the Brazilian Unified Health System (SUS). Some studies show that the public funding of phacoemulsification procedures by SUS is often insufficient to meet the surgeon’s training costs because of the greater number of complications and greater material consumption per surgery29. A 2010 study29 showed that there is a significant difference between the expenses in cataract surgeries performed by experienced surgeons and in those performed by residents in training, even when the residents are in the third and final year of training in the Brazilian Ophthalmology Program.
Surgeons in the learning phase have more complications in cataract surgeries, even when supervised by experienced staff, compared with surgeons who already master the phacoemulsification technique30. Although there is no standardization on how cataract surgery should be taught, some methods have been proposed to mitigate complications from the phacoemulsification learning curve. One example is learning this technique “backward,” i.e., dividing the surgery into specific steps, with the surgeon in training initially performing only the final steps, while the other steps are performed by a more experienced surgeon31. When the student becomes familiar with that step, he or she starts performing the previous step, and so on, until the student becomes familiar with the complete procedure. This methodology is based on the principle that the initial steps of phacoemulsification, such as capsulorhexis and nucleus fracture, are the most critical for the development of the procedure, and they should not be performed by a surgeon that is still not comfortable with the intraocular environment. Other schools advocate the initial learning of extracapsular surgery, which promotes prior familiarization with the ocular surgical environment and ophthalmic surgical equipment32 and can be used as an alternative rescue surgery technique in cases of complicated phacoemulsification. After being able to perform extracapsular surgery and becoming familiar with the microsurgical apparatus, the resident can then migrate to phacoemulsification surgery.
In addition to direct teaching in the operating room, other forms of education in cataract surgery are widely used to optimize the surgical learning curve. Wet labs, dry labs, and virtual simulators are parts of the new teaching arsenal in ophthalmology. The availability of a structured competency-based curriculum with formative feedback to residents, as opposed to the traditional curriculum with only lectures and theoretical tests, can reduce complications. This finding was demonstrated by Rogers at the Veterans Affairs Medical Center service in Iowa; the study showed a statistically significant reduction in cataract surgery complications, from 7.17% before implementing the curriculum changes to 3.77% on following the optimized curriculum33.
In Brazil, the National Council of Medical Residency (CNRM) resolution No. 02/2006 states that each ophthalmology resident must perform at least 50 surgeries in every year of the residency program. However, the resolution does not specify what type of surgeries should be performed during the residency34. In the United States, the Accreditation Board for Graduate Medical Education requires residents to perform at least 86 cataract surgeries during the three years of specialization35. Some studies show that the number of complications associated with cataract surgeries performed by residents decreases about 50% after performing surgeries in 40 cases and becomes acceptable after approximately 100 cases36. History shows that even more than two millennia after the initiation of cataract surgery, the equipment, materials, and surgical techniques are still constantly developing. In addition to this constant change, the challenge of teaching cataract surgery techniques involves the need for access to training centers in all regions. Surgeons in training require more financial resources and qualified supervision; however, a large portion of the population that still loses the essential function of vision because of a disease that is completely reversible surgically can benefit from gaining access to ophthalmologists who are adequately prepared to perform cataract surgery.
REFERENCES
1. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614-8.
2. Avicenna, Al-Qanun fi’l-tibb. In: Hesan Ja`far, editor. Al-Hilal. Beirut: Dar-ol- Behar, 2009.
3. Avicenna, The Canon (Persian translation), 3rd edition. Tehran: Ministry of Health and Medical Education of Iran, Committee of Computerizing Medicine and Hygiene, 2007.
4. Emery JM, McIntyre DJ. History of extracapsular cataract surgery. In: Emery JM, McIntyre DJ, eds. Extracapsular cataract surgery. St.Louis: C.V. Mosby, 1983.
5. Nejabat M, Maleki B, Nimrouzi M, Mahbodi A, Salehi A. Avicenna and cataracts: a new analysis of contributions to diagnosis and treatment from the canon. Iranian Red Crescent Med J. 2012;14(5):265-70.
6. Hubbell AA. Jacques Daviel and the beginnings of the modern operation of extraction of cataract. JAMA 1902;39:177-85.
7. Wyklicky H, Skopec M. Carl Koller and his time in Vienna. Regional anaesthesia. In: Scott DB, McClure J, Wildsmith JAW, eds. Regional Anaesthesia 1884Y1984. Sweden: ICM AB; 1984:12Y17.
8. Honegger H, Hessler H. Die Entdeckung der Lokalana ̈sthesie. Klin Monbl Augenheilkd Augena ̈rztl Fortbild. 1970;157:428Y438; 569Y578, 714Y723.
9. Davis G. The Evolution of Cataract Surgery. Mo Med 2016;113(1): 58-62.
10. Isawumi MA, Kolawole OU, Hassan MB. Couching techniques for cataract treatment in Osogbo, Southwest Nigeria. Ghana Med J. 2013;47(2):64-9.
11. Grzybowski A, Ascaso F. Sushruta in 600 B.C. Introduced Extraocular Expulsion of Lens Material. Acta Ophthalmol. 2014; 92(2):194-7.
12. Smith H. The treatment of cataract and some other common ocular affections. 2nd edition. Calcutta: Butterworth & Co, 1928.
13. Kirby D. History of cataract surgery. In: Surgery of cataract. Philadelphia: J.B. Lippincott, 1950.
14. Blodi FC. Cataract surgery. In: Albert DM, Edwards DD, eds. The history of ophthalmology. Oxford: Blackwell Science, 1996.
15. Apple DJ, Sims J. Harold Ridley and the invention of the intraocular lens. Surv Ophthalmol. 1996;40(4):279-92.
16. Nordlohne ME. The intraocular implant lens development and results with special reference to the Birkhorst lens. 2nd ed. Baltimore: Williams and Wilkins Company, 1975.
17. Kelman CD. Phacoemulsification and aspiration. A new technique of cataract removal. A preliminary report. Am J Ophthalmol. 1967;64(1):23-35.
18. Kelman CD. The History and Development of Phacoemulsification. Int Ophthalmol Clin. 1994;34(2):1-12.
19. Kelman CD. Phacoemulsification and aspiration. A progress report. Am J Ophthalmol. 1969;67(4):464-77.
20. Linebarger EJ, Hardten DR, Shah GK, Lindstrom RL. Phacoemulsification and Modern Cataract Surgery. Surv Ophthalmol. 1999;44(2):123-47.
21. Martin AI, Sutton G, Hodge C. The Evolution of Cataract Surgery: Controversies through the ages. Asia Pac J Ophthalmol (Phila). 2013;2(4):213-6.
22. Song E, Li X, Bi MC, Ren H, Wang D, Cui ZH, et al. A comparison of surgical efficacy between a 1.8-mm microincision and 3.2-mm and 5.5-mm incisions for phacoemulsification. Int J Ophthalmol. 2018;11(3):516-9.
23. Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16(1):31-7.
24. Jaffe NS. History of Cataract Surgery. Ophthalmology. 1996;103: S5-S16.
25. Kershner RM. Retinal image contrast and functional visual performance with aspheric, silicone, and acrylic intraocular lenses. Prospective evaluation. J Cataract Refract Surg. 2003; 29(9):1684-94.
26. Novis C. Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol. 2000;11(1):47-50.
27. Bohm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: Diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J Cataract Refract Surg. 2019;45(11):1625- 36.
28. Kanclerzk P, Alio JL. The benefits and drawbacks of femtosecond laser- assisted cataract surgery. Eur J Ophthalmol. 2020; 1120672120922448.
29. Carricondo PC. Análise dos custos e complicações da cirurgia de catarata realizada por residentes. 2010. Tese de Doutorado. Universidade de São Paulo.
30. Taravella MJ, Davidson R, Erlanger M, Guiton G, Gregory D. Characterizing the learning curve in phacoemulsification. J Cataract Refract Surg. 2011;37(6):1069-75.
31. Suryawanshi M, Gogate P, Kulkarni AN, Biradar A, Bhomaj P. Comparison of the Posterior Capsule Rupture Rates Associated with Conventional (Start to Finish) Versus Reverse Methods of Teaching Phacoemulsification. Middle East Afr J Ophthalmol. 2016;23(2):163-7.
32. Soriano ES. Cataract surgery teaching. Arq Bras Oftalmol. 2015; 78(4):V-VI.
33. Rogers GM, Oetting TA, Lee AG, Grignon C, Greenlee E, Johnson AT, et al. Impact of a structured surgical curriculum on ophthalmic resident cataract surgery complication rates. J Cataract Refract Surg. 2009;35(11):1956-60.
34. Resolução CNRM No 02 /2006, disponível em: http://portal.mec.gov.br/dmdocuments/resolucao02_2006.pdf.
35. Rowden A, Krishna R. Resident cataract surgical training in United States residency programs. J Cataract Refract Surg. 2002; 28(12):2202-5.
36. Ament CS, Henderson BA. Optimizing resident education in cataract surgery. Curr Opin Ophthalmol. 2011;22(1):64-7.
AUTHOR’S INFORMATION
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
November 13, 2020.
Accepted on:
December 21, 2020.