Nayara Queiroz Cardoso Pinto1; Lorena Maria Araújo Gomes1; Renan Magalhães Montenegro Júnior1; Sarah Diógenes Alencar2; Marcelo Bezerra Diógenes3; Raquel Diógenes Alencar4
DOI: 10.17545/eOftalmo/2019.0030
ABSTRACT
This article aims to report a case of cytomegalovirus retinitis in a patient who underwent renal transplant and was treated with intravenous ganciclovir after diagnosis. However, owing to adverse reactions, this medication had to be discontinued, and an intravitreal dose of ganciclovir was administered instead. The patient showed little clinical improvement after initiation of intravenous therapy, and the intravitreal dose was given as replacement. The patient responded extremely well to the treatment. She showed good visual recovery and improvement on color retinography and optical coherence tomography of the affected eye. The contralateral eye showed no alterations. Cytomegalovirus retinitis is a rapidly evolving disease that can cause severe ocular sequelae; early detection and treatment of this disease result in better visual prognosis. The use of intravitreal ganciclovir allows a good vitreous concentration and a good response in most patients, as reported previously. It is an excellent tool for vision recovery, especially in patients in whom interruption of the intravenous drug administration is necessary.
Keywords: Ganciclovir; Cytomegalovirus; Renal transplant; Retinitis; Eye.
RESUMO
Relatar o caso clínico de retinite por citomelovírus em uma paciente transplantada renal que após o diagnóstico foi tratada com ganciclovir endovenoso, entretanto devido a reações adversas a medicação teve que ser suspensa e optou-se pela dose intravítrea de ganciclovir. Após o início da terapia intravenosa a paciente tinha possuído pouca melhora clínica e após a suspensão da medicação optou-se pela dose intravítrea. A paciente respondeu de forma excelente ao tratamento. Na evolução apresentou boa recuperação visual e melhora no aspecto da retinografia colorida e da tomografia de coerência óptica do olho acometido. O olho contralateral não apresentou alterações. A retinite por citomegalovírus é uma doença de rápida evolução e que pode provocar sequelas oculares graves. A detecção e o tratamento precoce resultam em melhor prognóstico visual. O uso do ganciclovir intravítreo possibilita uma boa concentração vítrea e uma boa resposta na maioria dos pacientes relatados, sendo seu uso uma excelente ferramenta de auxílio na recuperação da visão, principalmente nos casos de necessidade de interrupção da medicação intravenosa.
Palavras-chave: Ganciclovir; Citomegalovírus; Transplante de rim; Retinite; Olho.
INTRODUCTION
Cytomegalovirus (CMV) is a member of the viral family known as herpes virus, herpesviridae, or human herpesvirus-5(1). It is usually asymptomatic or presents with mild symptoms in immunocompetent patients. The conditions most related to its clinical manifestation occur in immunocompromised patients, secondary to cell-mediated immunity depression (with CD4 counts of <50/mm3), being more frequently associated with acquired immunodeficiency syndrome (AIDS), use of immunosuppressive drugs (e.g., autoimmune diseases and organ transplantation), blood transfusion, and lymphoma(1-3).
The most commonly described eye involvement associated with CMV is retinitis, being the most common opportunistic ocular infection in patients with AIDS, affecting 6%-38% patients(4). In a study comprising 860 patients who underwent transplantation, only 19 (2%) were identified to have eye complications, 65% of these being opportunistic infections. Herpetic viral retinitis (77%) and fungal chorioretinitis (22%) were observed, but the viral infection was the most severe eye involvement, being the most common in the second year after transplantation(5).
Symptoms may vary according to the site of retinal involvement; when it occurs in the peripheral region, the patient may be asymptomatic. Patients begin to complaint regarding issues pertaining the eyes as the infection progresses. Generally, there is a mild anterior chamber reaction (ACR), as well as in the vitreous. Eventually, classic and fulminant presentation of hemorrhagic necrotic retinitis will appear on the periphery and may affect the posterior pole. Frosted angiitis may appear in areas with and without retinitis. Venous occlusive disease and neovascularization of the optic disc are less common(4).
Because visual complaints are not always present at the beginning of the condition and diagnosing the condition late may favor severe ocular sequelae and worse visual prognosis, it is essential to screen patients with risk factors(9). In a study involving 120 patients with CMV retinitis, blurred vision and loss of vision (<20/40) were associated with CMV in only 29% of the 16 patients diagnosed(6).
An ophthalmological examination is mandatory for all patients with AIDS having CD4 counts of <100/mm3. Regarding immunosuppressive drugs, higher the dose and longer the period, higher is the risk of developing CMV retinitis; patients with such conditions should also be accompanied, mainly in situations where some other opportunistic disease has settled(6-8).
The classically adopted treatment is performed with intravenous ganciclovir (IVG), which has the benefit of systemic control and reduction of CMV and mortality rate, but has pancytopenia as the main complication. Alternatively, intravitreal injection of ganciclovir (IVTG) is an easy and safe method of rapidly delivering high intraocular concentrations of the drug(10).
CASE REPORT
M.J.S, a 45-year-old female patient, underwent renal transplant secondary to IgA nephropathy. She was referred to the ophthalmology service of Walter Cantídio University Hospital in November 2016 owing to complaints of reduced vision in the right eye for one month and intravenous use of ganciclovir, in an attack dose, corrected by creatinine clearance two weeks earlier.
During the examination, she presented 20/60 corrected visual acuity in the right eye (OD) and 20/20 in the left eye (OS). Biomicroscopy revealed fine keratic precipitates and 1+/4+ ACR, which was phasic in the OD and no changes in the OS. The intraocular pressure (IOP) was normal in both eyes, and funduscopic examination revealed a blurred optic nerve, a whitish retinal lesion with imprecise boundaries, and retinal hemorrhages near the lower temporal arch in the OD (Figures 1A, 1C, and 2A). There were no changes in the OS (Figures 1B and 1D).
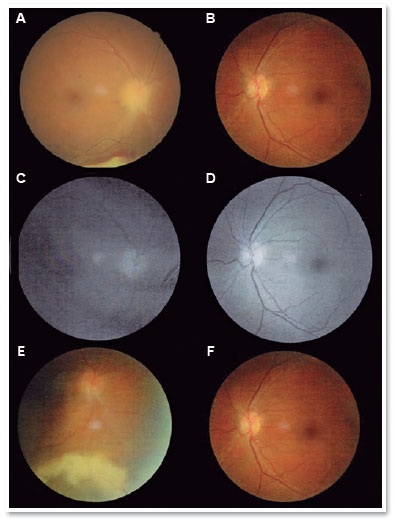
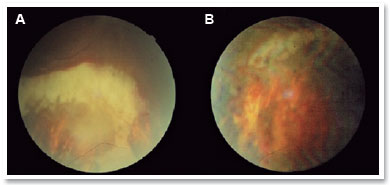
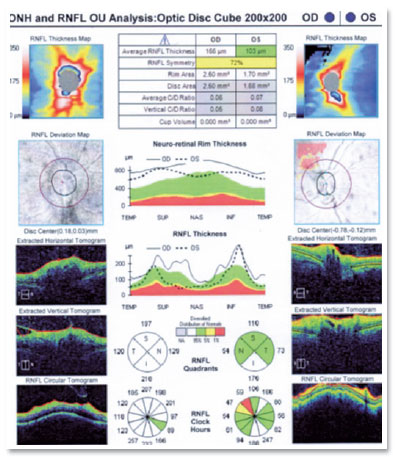
Optical coherence tomography (OCT) of the macula presented attenuation of the foveal depression and increased retinal thickness, with the presence of small hyporreflective spaces in the OD and presence of optic nerve edema (Figure 3). IVG attack dose was maintained, and the patient was re-evaluated 15 days later; however, the lesion still showed signs of activity, and the patient developed persistent leukopenia. Then, IVTG was discontinued, and 2 IVTG were administered at a dose of 2mg/0.1ml, with an interval of 15 days. Fifteen days after the last application, she had 20/25 corrected visual acuity, without RCA; optic nerve with more defined edges retinal lesion with regular boundaries, with cicatricial appearance; and no bleeding in the OD (Figures 1E and 2B). The OCT of the macula showed physiological foveal depression and absence of hyporreflective spaces (Figure 4). After 3 months of IVTG, there were no signs of relapse.
DISCUSSION
CMV retinitis is an example of a retinal response to a low virulence organism in an immunocompromised host. Patients who have undergone transplantation are at risk of developing CMV retinitis owing to the use of immunosuppressive medications. In a study comprising 25 patients who had undergone kidney transplantation, 21 presented some ocular alteration, mainly cataracts (15) and retinal pigment epithelium depigmentation (5), whereas others had increased IOP (4) and glaucoma (1). The most severe ocular alteration reported was CMV retinitis in two cases(7).
In another study, ocular complications of 39 patients who underwent kidney transplantation were evaluated; of these, nine (23%) had early posterior subcapsular cataract, two (5%) developed CMV retinitis (CMVR), and one developed steroid-induced glaucoma. The eye lesions found were all attributable to the use of immunosuppressants. Thirty-five (87%) had complement fixative antibodies to CMV(8). Among patients with VCMV, in one patient, the infection advanced rapidly into both eyes, caused great retinal damage, and evolved into legal blindness. The second patient presented with severe visual impairment in one eye. Ten of these patients had high titers of antibodies against CMV; most importantly, six exhibited an increase in the antibody titers during the observation period; however, only two patients developed CMVR.
IVTG is the most common treatment for CMVR, but because it is only virostatic, maintenance therapy is necessary to prevent relapse of the infection. In up to 12%-20% patients, infections may not resolve with induction therapy; there were relapses in many patients at an average duration of approximately 8 weeks during maintenance therapy. Another challenge with the use of ganciclovir is its toxic effect, as observed in the patient described here. An intravitreal injection is a good option in such patients, with an advantage of supplying the drug directly to the posterior eye segment and avoiding major systemic reactions(11-12).
IVTG is associated with the risk of endophthalmitis, vitreous bleeding, retinal vascular occlusions, and retinal detachment (this risk increases with increasing number and frequency of injections). The reported dose is 0.2-5mg of ganciclovir, and the recommended injection administration schedule is two weekly doses for 2 weeks, maintained weekly until the patient’s immune system reaches a state of reconstitution, and the lesion becomes a scar(10). In the case reported, the patient presented a good response with only two doses of the injection, being in a scar state and stable for the 12-week monitoring period. Results of another study showed that intravitreal ganciclovir administration at a dose of 2mg/0.1ml has good efficacy and has been used in 35 eyes of 22 patients with CMVR, of which only two patients relapsed during the maintenance therapy with weekly injections at 25 and 42 weeks(11).
CMVR is a rapidly evolving disease that can cause severe ocular sequelae; early detection and treatment result in better visual prognosis. IVG results in a good response in these patients, preventing contralateral eye involvement, systemic or visceral involvement, and decreasing mortality. IVTG enables a good vitreous concentration and a good response in most reported patients, and it is an excellent aid tool in the recovery of vision, especially in those patients requiring interruption of intravenous medication (13). However, cataract induction and a possibility of retinal detachment are important potential complications demanding caution.
This case of a 45-year-old patient who underwent renal transplantation presented an excellent clinical and visual response to IVTG.
REFERENCES
1. Gupta M, Shorman M. Cytomegalovirus. Treasure Island (FL): StatPearls Publishing; 2019 Jan.
2. Hosseini SM, et al. Bilateral cytomegalovirus retinitis in a healthy infant. Journal of Current Ophthalmology, Elsevier BV. 2017;29(1):66-8.
3. Chawla R, et al. Treatment of unilateral zone I cytomegalovirus retinitis in acute lymphoblastic leukemia with oral valganciclovir and intravitreal ganciclovir. Oman Journal of Ophthalmology. 2017 Oct;10(3):250-2.
4. Yannuzzi, LA. Atlas de Retina. 1ª ed. Elsevier, Rio de Janeiro, 2010.
5. Ng P, McCluskey P, McCaughan G, et al. Ocular complications of heart, lung, and liver transplantation. Br J Ophthalmol. 1998 Apr;82:423-8.
6. Colby DJ, et al. Prevalence and predictors of cytomegalovirus retinitis in HIV-infected patients with low CD4 lymphocyte counts in Vietnam. International Journal of Std & Aids. 2013 Dec;25(7):516-22.
7. Berkowitz JS, et al. Ocular complications in renal transplant recipients. The American Journal of Medicine. 1973 Oct;55(4): 492-5.
8. Porter R, et al. Incidence of Ocular Complications in Patients Undergoing Renal Transplantation. Bmj. 1972 Jul;3(5819):133-6.
9. Liu Y, et al. Diagnostic Utility of Ocular Symptoms and Vision for Cytomegalovirus Retinitis. Plos One. 2016;11(10):e0165564.
10. Choopong P, et al. Treatment outcomes of reduced-dose intravitreal ganciclovir for cytomegalovirus retinitis. Bmc Infectious Diseases. 2016 Apr;16(1):1-7.
11. Morlet N, et al. High dose intravitreal ganciclovir injection provides a prolonged therapeutic intraocular concentration. Br J Ophthalmol. 1996;80(3):214-6.
12. Harris ML, Mathalone MB. Intravitreal ganciclovir in CMV retinitis: case report. Br J Ophthalmol. 1989;73(5):382-4.
13. Rabadão E, Duque V, Meliço-Silvestre A. The treatment of cytomegalovirus retinitis in human immunodeficiency virus infection. Acta Médica Portuguesa. 1999 Jun;12(4-6)203-7.
AUTHOR'S INFORMATION






Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
April 25, 2019.
Accepted on:
September 27, 2019.