Aline Mota Freitas Matos1; Gustavo Henrique de Lima Melillo2; Bianca Figueiredo Barczewski3
DOI: 10.17545/eoftalmo/2018.0033
ABSTRACT
The Langerhans’ Cell Histiocytosis (LCH) is a group of disorders caused by clonal proliferation and accumulation of these cells in various organs, such as skin, hematopoietic or lymphoid tissues, lungs, liver, hypothalamus and bones. The eyelid presentation is extremely rare. Here, we report a case of a skin-limited upper eyelid LCH. The 35-year-old woman presented solid crusted nodule on the left upper eyelid margin, causing the misdirection of some of the adjacent eyelashes with progressive growth for 1 year. Excisional biopsy was performed, and histological analysis revealed histiocytes with eosinophilic cytoplasm, immunohistochemical positivity for S-100, laminin and CD1a, which showed LCH. Though rare, the skin-limited LCH should be included in the differential diagnoses of tumoral lesions of the eyelid.
Keywords: Histiocytosis; Eyelid; Langerhans Cells.
RESUMO
A histiocitose de células de Langerhans (LCH) abrange um grupo de desordens caracterizadas pela proliferação clonal e acúmulo dessas células em vários órgãos como pele, tecidos hematopoiéticos ou linfóides, pulmões, fígado, hipotálamo e ossos. A apresentação de lesão palpebral é extremamente rara. Aqui, relatamos um caso de LCH de pálpebra superior limitada à pele. Uma paciente de 35 anos apresentou um nódulo sólido com crosta na margem da pálpebra superior esquerda, causando mal direcionamento de alguns dos cílios adjacentes com crescimento progressivo durante um ano. Biópsia excisional foi realizada e a análise histológica revelou histiócitos com citoplasma eosinofílico, positividade imunoistoquímica para S-100, laminina e CD1a, revelando LCH. Embora rara, a LCH limitada à pele deve ser incluída nos diagnósticos diferenciais de lesões tumorais palpebrais.
Palavras-chave: Histiocitosis; Pálpebras; Células de Langerhans.
RESUMEN
La histiocitosis de células de Langerhans (HCL) abarca un grupo de desórdenes caracterizados por la proliferación clonal y acúmulo de dichas células en varios órganos, como piel, tejidos hematopoyético o linfoide, pulmones, hígado, hipotálamo y huesos. La presentación de lesión palpebral es extremadamente rara. Aquí, relatamos un caso de HCL de párpado superior limitada a la piel. Una paciente de 35 años presentó un nódulo sólido con costra en el margen del párpado superior izquierdo, causando mal direccionamiento de algunas de las pestañas adyacentes con crecimiento progresivo durante un año. Se ha realizado la biopsia excisional y el análisis histológico ha indicado histiocitos con citoplasma eosinófilo, positividad inmunohistoquímica para S-100, laminina y CD1a, indicando HCL. Aunque rara, la HCL limitada a la piel debe incluirse en los diagnósticos diferenciales de lesiones tumorales palpebrales.
Palabras-clave: Histiocitose; Párpados; Células de Langerhans.
INTRODUCTION
Histiocytosis are macrophages and dendritic cells proliferative lesions and can cause a wide range of systemic disorders. Langerhans cells are an immature type of dendritic cells typically present in skin and mucosa that play an important role in immune responses1. The Langerhans’ Cell Histiocytosis (LCH), also called Histiocytosis X, is a group of disorders caused by clonal proliferation and accumulation of these cells in various organs, such as skin, hematopoietic or lymphoid tissues, lungs, liver, hypothalamus and bones. Tipically described in children, its course ranges from benign spontaneous involution to systemic failure2. The eyelid presentation has rarely been reported, few of them in adults3. We present herein a case of this uncommon eyelid margin diagnose.
CASE REPORT
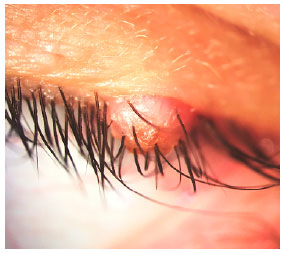
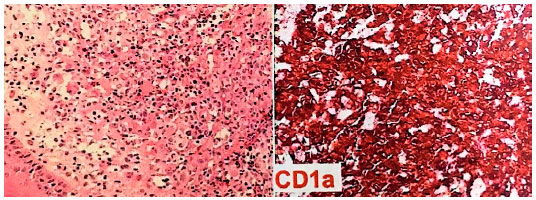
Female, 35 years old, presented solid crusted nodule on the left upper eyelid margin (Figure 1), causing the misdirection of some of the adjacent eyelashes. The lesion had been growing for 1 year. The patient denied other similar lesions along the body and, except for hypothyroidism, she did not report any personal or familial antecedents of interest. BCVA 20/20 in both eyes. Other ophthalmic examination results were within normal ranges. Excisional biopsy was performed. The anatomopathological analysis revealed histiocytes with eosinophilic cytoplasm, immunohistochemical stains for S-100, laminin and, more remarkably, positivity to CD1a (Figure 2). Given those findings, the diagnosis of LCH was made.
DISCUSSION
Histiocytes are cells of the reticuloendothelial system and include macrophages, dendritic cells and Langerhans cells (LC). The LC are usually charachterised by the presence of Bierbeck granules, organelles supposedly involved in antigen internalization4. LC normally occur in body surface, such as epidermis, conjunctiva and corneal limbus. They are rare in healthy tarsal conjunctiva. However, LC are numerous in subconjunctival tissue and eyelid margin skin of patients with allergic conjunctivitis5.
Nodular lesions of the eyelid are among the most frequent abnormalities of the periocular area. The base of the eyelashes is an usual spot for eccrine hydrocistoma, chalazion, sebaceous cyst, siringoma, xanthelasma, epidermal inclusion cyst and malignant tumors. Differential diagnosis is paramount for the correct treatment and follow-up and is made both clinically and histologically. The LCH however, is exceptionally located in this site. We were not able to find more than 6 case reports of the tumor in the eyelid, and only one in the upper [3,4,6-9].
The LCH was first desribed in 1893 by Alfred Hand Jr, who observed exophthalmia, hepatoesplenomegaly, lymphadenopathy and diabetes insipidus. Throughout the twentieth century, other contributors shed light on the various aspects of the disease, although the nature of the physiopathology is yet to be unveiled2. As the pathogenesis is poorly understood, different origins for the disease have been postulated. Viral infection, immune dysfunction and malignancy have already been suggested as mechanisms for the development of the disease. Some association with thyroid disease have also been demonstrated4. Recently, the discovery of frequent oncogenic BRAF mutations in LCH supports its classifictation as neoplasm10.
The peak of incidence of LCH occurs between 1-4 years, however, it may present at all ages. With an estimated incidence of 4.0-5.4 cases per million children, its epidemiological data is difficult to attain due to the rarity of the disease11. In addition, LCH seems to be underdiagnosed as it can be symptomless or mistaken for other disorders12.
The LCH affects most frequently the osseous, pulmonary, mucocutaneous, thalamic and lymphatic tissues13. In the ophthalmic practice, the disorder involves more frequently in the orbit, accouting to less than 1% of the orbital tumefactions. The typical presentation is of a slowly growing upper palpebral swelling over a period of weeks to months. Acute presentation is rare, usually appearing due to inflammatory response to the lesion, and can be confused with periorbital cellulitis or dacryoadenitis. Proptosis, nerve palsies, papilledema and cavernous sinus syndrome can be found in orbital disease14. The occurrence of LHC in the lacrimal gland of the adult was reported15. The eyelid is rarely affected.
Histological examination reveals cells with abundant eosinophilic cytoplasm, and background eosinophils, lymphocytes, histiocytes and neuthrophils present in variable quantities. The immunophenotype of LCH includes expression of CD1a, S100 protein and langerin (CD207). The differential diagnosis includes Langerhans cell sarcoma, other histiocytc lesions and lymphoma. The immunohistochemical and morphologic features described are generally enough to diagnose16.
The multisystem disease treatment is protocol based and dependent on a number of variables. Complete excision of the lesion may be curative for focal cutaneous disease. Steroids, nitrogen mustard of psoralen combined with ultraviolet A (PUVA) are second-line options. Radiation therapy is an option when the lesions are recurrent or progressive4. The lesions may progress to chronic or relapsing process with spontaneous remission or treatment. Skin-limited LCH has excelent overall prognosis17.
As this case report demonstrates, the LCH can be skin-limited and found in exceptional locations as the eyelid. Thus, it should be considered as a differential diagnose towards tumoral lesions of the eyelid.
REFERENCES
1. Kumar V, Abbas AK, Fausto N, Mitchell R. Robbins & Cotran: Patologia. Rio de Janeiro: Elsevier; 2008.
2. Néel A, Artifoni M, Donadieu J, Hamidou M, Tazi A. Histiocytose langerhansienne de l’adulte. La Revue de Médecine Interne. 2015; 36:658-67.
3. Ramzan M, Yadav SP, Bhalla S, Jamwal P, Grover AK, Sachdeva A. Eyelid nodule: a rare presentation of langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2012; 34:c158-c160.
4. Leveson J, Bourque JM, Lukovic J, Rashid Dar A. Radiotherapy for langerhans cell histiocytosis of bilateral eyelids. Cureus. 2016 Feb; 8(2):e474.
5. Takaura N, Inada N, Shoji J, Sawa M. Distribution of S-100 protein positive cells in conjunctiva. Nippon Ganla Gakkai Zasshi. 1995; 99:873-7.
6. Chikama T, Yoshino H, Nishida T, et al. Langerhans cell histiocytosis localized to the eyelid. Arch Ophthalmol. 1998; 116:1375-7.
7. Weissgold DJ, Wulc AE, Young M, et al. Eosinophilic granuloma of the eyelid. Ophthal Plast Reconstr Surg. 1994; 10:160-2.
8. Tosaka Y. A case of localized histiocytosis X of the eyelid. Nippon Ganka Gakki Zasshi. 1989; 93:103-8.
9. Daras C, Grayson W, Mayet I, et al. Langerhans’ cell histiocytosis of the eyelid. Br J Ophthalmol. 1995; 79:91-2.
10. Badalian-Very G, Vergilio JA, Fleming M, Rollins BJ. Pathogenesis of Langerhans cell histiocytosis. Annu Rev Pathol. 2013 Jan; 8:1-20.
11. Nicholson HS, Egeler RM, Nesbit ME. The Epidemiology of Langerhans cell histiocytosis. Hematol Oncol Clon Noth Am. 1988 Apr; 12(2):379-84.
12. Egeler RM, D’Angio GJ. Langerhans cell histiocytosis. J Pediatr. 1995 Jul; 127(1):1-11.
13. Howarth DM, Girlchrist GS, Mullan BP, et al. Langerhans cell Histiocytosis. Cancer. 1999; 85:2278-90.
14. Levy J, Monos T, Kapelushnik J, Maor E, et al. Ophthalmic manifestations in Langerhans cell histiocytosis. IMAJ. 2004; 6:553-5.
15. Pace ST, Gelman BB, Wong BR. Primary Langerhans cell histiocytosis of the lacrimal gland in an adult. Can J Ophthalmol. 2015 Jun; 50(3):e40-3.
16. Harmon CM, Brown N. Langerhans cell histiocytosis: a clinicopathologic review and molecular pathogenetic update. Arch Pathol Lab Med. 2015 Oct; 139(10):1211-4.
17. Ehrhardt MJ, Humphrey SR, Kelly ME, Chiu YE, Galbraith SS. The natural history of skin-limited langerhans cell histiocytosis: a single-institution experience. J Pediatr Hematol Oncol. 2014; 36:613-6.


Funding: No specific financial support was available for this study
CEP Approval: Not applicable
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
June 19, 2018.
Accepted on:
August 30, 2018.